Introduction
Zinc, a transition metal with atomic number 30, plays a crucial role in various biological processes. Its divalent cation, Zn2+, is particularly important in enzyme catalysis, cellular signaling, and immune function. Understanding the electron configuration of Zn2+ is essential for unraveling its chemical properties and biological significance.

Zn2+ Electron Configuration: A Closer Look
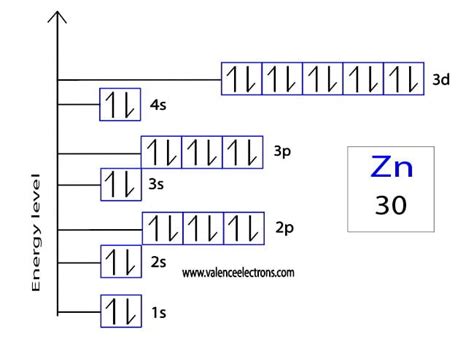
The electronic configuration of Zn2+ can be derived from the neutral zinc atom. Zinc has 30 electrons, distributed in its energy levels as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²
When zinc loses two electrons to form Zn2+, the outermost 4s electrons are removed, resulting in the following electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰
Electronic Properties of Zn2+
The electron configuration of Zn2+ reveals several important electronic properties:
- D¹⁰ Configuration: The stability of Zn2+ arises from its d¹⁰ configuration, which has a completely filled d orbital. This configuration imparts inertness to the ion, making it less likely to participate in chemical reactions involving d-electron exchange.
- High Ionization Energy: The removal of electrons from the filled d¹⁰ configuration requires a significant amount of energy, which contributes to the stability of Zn2+.
- Low Redox Potential: Zn2+ has a low redox potential, indicating its preference for the +2 oxidation state. This low redox potential allows Zn2+ to participate in redox reactions without undergoing substantial oxidation or reduction.
Biological Significance of Zn2+
The unique electronic properties of Zn2+ make it an essential cofactor for numerous biological enzymes. Over 300 enzymes rely on Zn2+ for their catalytic activity, including:
- Carbonic anhydrase: Facilitates the conversion of carbon dioxide to bicarbonate ions.
- Superoxide dismutase: Scavenges superoxide radicals, protecting cells from oxidative damage.
- Alcohol dehydrogenase: Catalyzes the oxidation of alcohols.
Applications of Zn2+
Beyond biological systems, Zn2+ finds applications in various fields, including:
- Industrial Processes: Zn2+ is used in electroplating, galvanizing, and the production of zinc alloys.
- Paints and Pigments: Zinc oxide (ZnO) is a white pigment widely employed in paints, coatings, and sunscreens.
- Batteries: Zinc-air batteries utilize the oxidation of Zn2+ to generate electricity.
Conclusion
The electron configuration of Zn2+ provides insights into its electronic structure, stability, and biological significance. With its d¹⁰ configuration, high ionization energy, and low redox potential, Zn2+ plays a crucial role in enzyme catalysis and other biological processes. Its versatility extends to industrial and technological applications, making it an essential element in various domains.