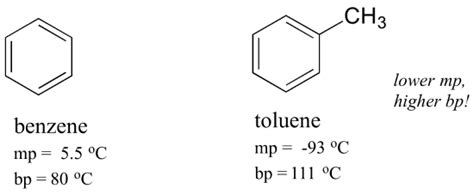
Toluene is a colorless, flammable liquid hydrocarbon with the chemical formula C7H8. It is a constituent of gasoline, paint thinners, and other solvents. Toluene has a boiling point of 110.6 °C (231.1 °F), which is higher than that of other hydrocarbons with similar molecular weights.

Factors Affecting Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure of the surrounding gas. The boiling point of a liquid is affected by several factors, including:
- Molecular weight: Heavier molecules have higher boiling points than lighter molecules.
- Polarity: Polar molecules have higher boiling points than nonpolar molecules.
- Intermolecular forces: Liquids with strong intermolecular forces have higher boiling points than liquids with weak intermolecular forces.
Toluene’s High Boiling Point
Toluene has a higher boiling point than other hydrocarbons with similar molecular weights because it has a relatively high polarity and strong intermolecular forces.
Polarity: Toluene is a polar molecule because it has a slightly positive carbon atom and a slightly negative hydrogen atom. The polarity of toluene is due to the difference in electronegativity between carbon and hydrogen. Electronegativity is a measure of an atom’s ability to attract electrons. Carbon is more electronegative than hydrogen, so it attracts the electrons in the C-H bond more strongly than hydrogen does. This creates a partial positive charge on the carbon atom and a partial negative charge on the hydrogen atom.
Intermolecular forces: Toluene has strong intermolecular forces because it is a nonpolar molecule. Nonpolar molecules are attracted to each other by van der Waals forces. Van der Waals forces are weak intermolecular forces that are caused by the temporary fluctuations in the electron distribution of a molecule. These fluctuations create instantaneous dipoles, which are temporary positive and negative charges on the molecule. The instantaneous dipoles of nonpolar molecules can attract each other, causing the molecules to stick together.
The polarity of toluene and its strong intermolecular forces contribute to its high boiling point.
Applications of Toluene
Toluene is used in a variety of applications, including:
- Gasoline: Toluene is a major component of gasoline. It is added to gasoline to increase its octane rating. Octane rating is a measure of a gasoline’s resistance to knocking. Knocking is a problem that can occur in internal combustion engines when the air-fuel mixture ignites too early. Toluene helps to prevent knocking by increasing the octane rating of gasoline.
- Paint thinners: Toluene is used as a paint thinner. It helps to dissolve paint and make it easier to apply.
- Other solvents: Toluene is used as a solvent in a variety of other applications, including the production of dyes, plastics, and pharmaceuticals.
Conclusion
Toluene has a high boiling point because it has a relatively high polarity and strong intermolecular forces. These properties make toluene a useful solvent and a major component of gasoline.