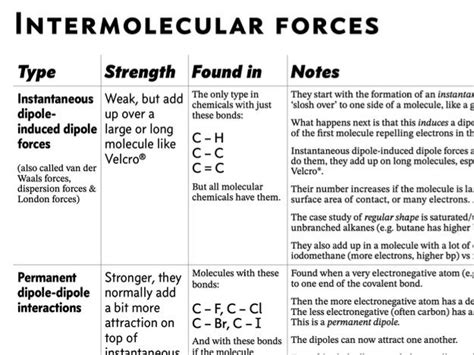
Intermolecular forces play a crucial role in determining the physical and chemical properties of molecules. They hold molecules together, affecting their behavior and interactions. Here’s a detailed analysis of the intermolecular forces present in various molecules:

Hydrogen Bonding
- Definition: A strong dipole-dipole interaction between a hydrogen atom covalently bonded to an electronegative atom (F, O, or N) and another electronegative atom.
-
Examples:
- Water (H2O): Exhibits strong hydrogen bonding between its electronegative oxygen atom and hydrogen atoms.
- Ammonia (NH3): Hydrogen bonds form between the hydrogen atoms of NH3 and the electronegative nitrogen atom.
Dipole-Dipole Interactions
- Definition: Attractive forces between polar molecules that possess permanent dipoles. These permanent dipoles arise due to differences in electronegativity between atoms within a molecule.
-
Examples:
- Carbon dioxide (CO2): The oxygen atoms in CO2 exert a stronger pull on electrons than the carbon atom, creating a permanent dipole.
- Chloroform (CHCl3): The polar covalent bonds between carbon and chlorine atoms result in a net dipole moment.
London Dispersion Forces
- Definition: Weak attractive forces that exist between all molecules, regardless of their polarity. They arise from the temporary fluctuations in electron distribution, creating instantaneous dipoles.
-
Examples:
- Nonpolar molecules like methane (CH4) and hexane (C6H14) primarily exhibit London dispersion forces due to their lack of permanent dipoles.
- Larger molecules generally have stronger London dispersion forces due to their increased surface area and more electrons.
Ion-Dipole Interactions
- Definition: Attractive forces between ions and polar molecules. Ions are charged species, while polar molecules possess a permanent dipole.
-
Examples:
- Sodium chloride (NaCl) dissolved in water: The sodium ions (Na+) and chloride ions (Cl-) interact with the water molecules (H2O) through ion-dipole forces.
- Ethanol (C2H5OH) mixed with water: The polar ethanol molecules interact with the sodium ions present in salt water.
| Molecule | Intermolecular Forces |
|---|---|
| Water (H2O) | Hydrogen bonding |
| Carbon dioxide (CO2) | Dipole-dipole interactions |
| Methane (CH4) | London dispersion forces |
| Sodium chloride (NaCl) in water | Ion-dipole interactions |
Applications of Intermolecular Forces
Understanding intermolecular forces is crucial for various applications across science and engineering. Some notable examples include:
- Drug Design: Intermolecular forces play a significant role in the interactions between drugs and their target sites. By designing drugs that utilize specific intermolecular forces, researchers can improve drug efficacy.
- Materials Science: The strength and type of intermolecular forces determine the properties of materials. By manipulating these forces, scientists can create new materials with tailored properties for various applications.
- Biochemistry: Intermolecular forces contribute to the structure and function of biological molecules. These forces stabilize proteins, nucleic acids, and other biomolecules, allowing them to carry out essential biological processes.
| Application | Intermolecular Force |
|---|---|
| Drug Design | Hydrogen bonding, dipole-dipole interactions |
| Materials Science | London dispersion forces, dipole-dipole interactions |
| Biochemistry | Hydrogen bonding, dipole-dipole interactions, ion-dipole interactions |
Additional Information
In addition to the discussed intermolecular forces, other weak interactions can influence molecular behavior, including:
- π-π Interactions: Attractive forces between the π-electron clouds of adjacent aromatic rings.
- Cation-π Interactions: Interactions between a cation and the π-electron cloud of an aromatic ring.
- Metal-Ligand Interactions: Interactions between metal ions and ligands through coordination bonds.
Conclusion
Intermolecular forces play a vital role in shaping the properties and interactions of molecules. Understanding these forces is crucial for various applications in science and engineering. By manipulating intermolecular forces, researchers can develop new materials, design more effective drugs, and unravel complex biological processes.