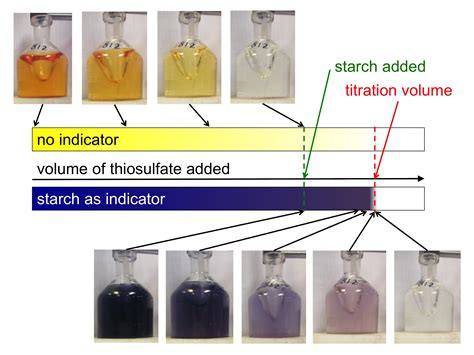
The concentration of oxygen in a sample can be measured using a variety of methods, including gas chromatography, mass spectrometry, and electrochemical sensors. The most common method is gas chromatography, which separates the components of a gas sample based on their boiling points. Once the components are separated, they are detected and quantified using a variety of detectors, including flame ionization detectors, thermal conductivity detectors, and mass spectrometers.

The concentration of oxygen in a sample is typically expressed in parts per million (ppm), parts per billion (ppb), or percent by volume (%v/v). The ppm unit is used to express the concentration of a gas in a mixture of gases, and the ppb unit is used to express the concentration of a gas in a mixture of liquids. The %v/v unit is used to express the concentration of a gas in a mixture of gases or liquids.
The concentration of oxygen in air is typically around 21%, but it can vary depending on the location and altitude. The concentration of oxygen in water is typically around 8 ppm, but it can vary depending on the temperature and salinity of the water. The concentration of oxygen in soil is typically around 10%, but it can vary depending on the type of soil and the presence of organic matter.
The concentration of oxygen in a sample is important for a variety of reasons. For example, the concentration of oxygen in air is important for human health, as humans need oxygen to breathe. The concentration of oxygen in water is important for aquatic life, as fish and other aquatic organisms need oxygen to survive. The concentration of oxygen in soil is important for plant growth, as plants need oxygen to respire.
How to measure the concentration of oxygen in a sample
The concentration of oxygen in a sample can be measured using a variety of methods, including:
- Gas chromatography
- Mass spectrometry
- Electrochemical sensors
The most common method is gas chromatography, which separates the components of a gas sample based on their boiling points. Once the components are separated, they are detected and quantified using a variety of detectors, including flame ionization detectors, thermal conductivity detectors, and mass spectrometers.
Applications of oxygen concentration measurement
The concentration of oxygen in a sample is important for a variety of applications, including:
- Human health
- Aquatic life
- Plant growth
- Industrial processes
- Environmental monitoring
The future of oxygen concentration measurement
The field of oxygen concentration measurement is constantly evolving, with new technologies being developed all the time. These new technologies are making it possible to measure the concentration of oxygen in a variety of samples with greater accuracy and precision.
FAQs
- What is the concentration of oxygen in air?
The concentration of oxygen in air is typically around 21%, but it can vary depending on the location and altitude.
- What is the concentration of oxygen in water?
The concentration of oxygen in water is typically around 8 ppm, but it can vary depending on the temperature and salinity of the water.
- What is the concentration of oxygen in soil?
The concentration of oxygen in soil is typically around 10%, but it can vary depending on the type of soil and the presence of organic matter.
- How can I measure the concentration of oxygen in a sample?
The concentration of oxygen in a sample can be measured using a variety of methods, including gas chromatography, mass spectrometry, and electrochemical sensors.
- What are the applications of oxygen concentration measurement?
The concentration of oxygen in a sample is important for a variety of applications, including human health, aquatic life, plant growth, industrial processes, and environmental monitoring.
- What is the future of oxygen concentration measurement?
The field of oxygen concentration measurement is constantly evolving, with new technologies being developed all the time. These new technologies are making it possible to measure the concentration of oxygen in a variety of samples with greater accuracy and precision.