Introduction
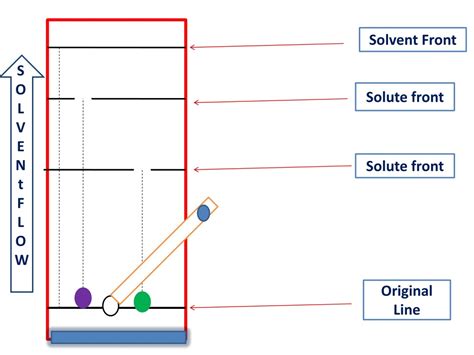
In paper chromatography and thin-layer chromatography (TLC), the solvent front refers to the boundary line between the stationary and mobile phases. It marks the furthest point reached by the solvent as it ascends the chromatographic medium (paper or TLC plate). Understanding the solvent front is crucial for analyzing and interpreting the results of these chromatographic techniques.

Definition and Significance
The solvent front is defined as the leading edge of the solvent as it moves through the chromatographic medium. It represents the point where the solvent has traveled the greatest distance from the origin. The rate at which the solvent front moves depends on several factors, including the nature of the solvent, the stationary phase, and the temperature.
The solvent front is significant because it determines the distance traveled by the analytes (substances being separated) on the chromatographic medium. The distance between the origin and the solvent front is known as the solvent migration distance. This distance is used to calculate the retention factor (Rf) of the analytes, which is a measure of their relative movement compared to the solvent.
Factors Affecting Solvent Front Migration
The rate at which the solvent front moves is influenced by various factors:
- Solvent: The polarity and volatility of the solvent are key factors. Polar solvents tend to move slower than nonpolar solvents, and volatile solvents move faster than less volatile solvents.
- Stationary phase: The polarity and texture of the stationary phase also affect solvent migration. Polar stationary phases attract polar solvents, slowing their movement, while nonpolar stationary phases have the opposite effect.
- Temperature: Higher temperatures generally increase the rate of solvent migration.
- Other factors: The sample size, humidity, and presence of impurities can also influence solvent front movement.
Applications of Solvent Front
The concept of solvent front has several applications in chromatography:
- Separation of analytes: The solvent front helps separate analytes based on their relative affinities for the stationary and mobile phases. Analytes with a higher affinity for the stationary phase move slower, resulting in lower Rf values.
- Qualitative analysis: By comparing the Rf values of the analytes with known standards, the identity of the analytes can be determined.
- Quantitative analysis: The area or intensity of the analyte spots on the chromatogram can be used to estimate their concentrations.
Innovative Applications
Emerging applications for the solvent front include:
- Microfluidics: Miniaturized devices can utilize the solvent front for rapid and efficient separation and analysis.
- Biosensors: The solvent front can be used to detect and quantify specific analytes in biological samples.
- Environmental monitoring: The solvent front can aid in the detection and identification of pollutants in soil and water samples.
Conclusion
The solvent front is a fundamental aspect of paper and thin-layer chromatography. Its position on the chromatographic medium provides valuable information for the separation and analysis of analytes. Understanding the factors affecting solvent front migration is crucial for optimizing chromatographic conditions and achieving accurate results. With advancements in technology, the concept of solvent front continues to find novel applications in diverse fields.
Frequently Asked Questions
What is the difference between the solvent front and the mobile phase?
The solvent front is the leading edge of the mobile phase as it moves through the chromatographic medium. The mobile phase is the solvent or mixture of solvents used to carry the analytes through the chromatography system.
How is the solvent front used to calculate the Rf value?
The Rf value is calculated as the ratio of the distance traveled by the analyte to the distance traveled by the solvent front. A lower Rf value indicates that the analyte has a higher affinity for the stationary phase.
What factors can cause the solvent front to move unevenly?
Uneven movement of the solvent front can be caused by factors such as variations in the stationary phase, the presence of impurities, or uneven sample application.
Tables
| Parameter | Effect on Solvent Front Migration |
|---|---|
| Solvent polarity | Polar solvents move slower |
| Stationary phase polarity | Polar phases slow down polar solvents |
| Temperature | Higher temperatures increase solvent migration rate |
| Sample size | Larger samples can slow down solvent front movement |
| Chromatographic Technique | Solvent Front | Applications |
|---|---|---|
| Paper chromatography | Moving boundary of solvent on paper | Separation of small molecules, qualitative analysis |
| Thin-layer chromatography | Moving boundary of solvent on a TLC plate | Separation of complex mixtures, quantitative analysis |
| High-performance liquid chromatography (HPLC) | Moving boundary of mobile phase in a column | Pharmaceutical analysis, environmental monitoring |
| Industry | Application of Solvent Front |
|---|---|
| Pharmaceutical | Analysis of drug purity and potency |
| Environmental | Detection and quantification of pollutants |
| Forensic science | Identification of substances in evidence samples |
| Innovative Application | Description |
|---|---|
| Microfluidic devices | Miniaturized chromatography for rapid analysis |
| Biosensors | Solvent front-based detection of analytes in biological samples |
| Environmental monitoring | Detection and identification of pollutants in soil and water |