When you see the symbol “Ag” in a reaction equation, it means that silver is involved in the reaction. Silver is a chemical element with the symbol Ag and atomic number 47. It is a soft, white, lustrous transition metal that has been known since ancient times. Silver is used in jewelry, coinage, photography, and electronics.

In a reaction equation, the symbol “Ag” can represent either silver atoms or silver ions. Silver atoms are neutral, meaning they have no net electrical charge. Silver ions have a positive electrical charge, meaning they have lost one or more electrons.
The most common silver ion is the silver(I) ion, which has a charge of +1. The silver(I) ion is formed when a silver atom loses one electron. The silver(II) ion, which has a charge of +2, is less common. The silver(II) ion is formed when a silver atom loses two electrons.
In a reaction equation, the symbol “Ag” can also represent silver compounds. Silver compounds are chemical compounds that contain silver atoms. Silver compounds can be either ionic or covalent. Ionic silver compounds contain silver ions, while covalent silver compounds contain silver atoms that are bonded to other atoms by covalent bonds.
The most common silver compound is silver chloride (AgCl). Silver chloride is a white, crystalline solid that is insoluble in water. Silver chloride is used in photography and in the production of silver jewelry.
Other common silver compounds include silver nitrate (AgNO3), silver sulfate (Ag2SO4), and silver oxide (Ag2O). Silver nitrate is a colorless, crystalline solid that is soluble in water. Silver nitrate is used in medicine and in the production of photographic film. Silver sulfate is a white, crystalline solid that is insoluble in water. Silver sulfate is used in the production of silver jewelry and in the purification of water. Silver oxide is a black, crystalline solid that is insoluble in water. Silver oxide is used in the production of silver batteries and in the purification of silver.
The Role of Silver in Chemical Reactions
Silver can participate in a variety of chemical reactions. Silver atoms can react with other atoms to form silver compounds. Silver ions can react with other ions to form silver compounds. Silver compounds can react with other compounds to form new compounds.
The most common type of chemical reaction that silver participates in is a redox reaction. A redox reaction is a reaction in which one or more atoms change their oxidation state. In a redox reaction, silver atoms can be either oxidized or reduced.
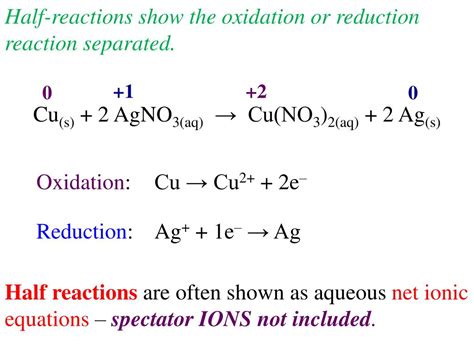
Oxidation is the loss of electrons. Reduction is the gain of electrons. When a silver atom is oxidized, it loses one or more electrons. When a silver ion is reduced, it gains one or more electrons.
The following are some examples of redox reactions that silver can participate in:
- Oxidation of silver atoms:
Ag → Ag+ + e-
- Reduction of silver ions:
Ag+ + e- → Ag
- Formation of silver compounds:
Ag+ + Cl- → AgCl
- Decomposition of silver compounds:
AgCl → Ag + Cl-
Applications of Silver
Silver has a wide variety of applications, including:
- Jewelry: Silver is a popular metal for jewelry because it is beautiful, durable, and relatively inexpensive. Silver jewelry is often adorned with gemstones or other metals.
- Coinage: Silver has been used to make coins for centuries. Silver coins are often more valuable than coins made of other metals, such as copper or nickel.
- Photography: Silver is used in the production of photographic film and paper. Silver halide crystals are used to capture light, which creates an image.
- Electronics: Silver is used in the production of electrical contacts, switches, and other electronic components. Silver is a good conductor of electricity and heat.
- Medicine: Silver is used in the production of medical devices, such as catheters and surgical instruments. Silver has antimicrobial properties, which helps to prevent infections.
Conclusion
Silver is a versatile metal with a wide range of applications. Silver is used in jewelry, coinage, photography, electronics, and medicine. Silver is also an important component of many chemical reactions.