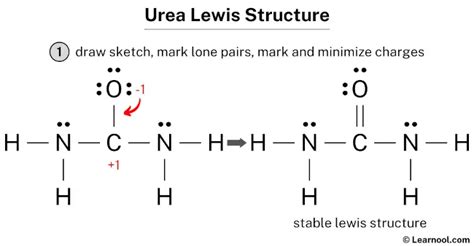
Understanding the Urea Lewis Structure
Urea, with the molecular formula CH₄N₂O, is an organic compound that plays a crucial role in various biological processes. Its Lewis structure provides valuable insights into its chemical bonding and properties.

The central atom in urea is nitrogen, surrounded by two hydrogen atoms, one carbon atom, and one oxygen atom. The Lewis structure of urea can be represented as:
H | H
| |
N - C - O
| |
H
Nitrogen shares two covalent bonds with hydrogen atoms and one covalent bond with each carbon and oxygen atoms, forming a tetrahedral geometry. The carbon atom is double-bonded to the oxygen atom and single-bonded to the nitrogen atom.
Key Features of the Urea Lewis Structure
- Polarity: Urea is a polar molecule due to the difference in electronegativity between nitrogen and the other atoms. The oxygen atom is the most electronegative, attracting electrons towards it and creating a partial negative charge. The nitrogen atom, being less electronegative, carries a partial positive charge.
- Hydrogen Bonding: Urea exhibits strong hydrogen bonding capabilities. The nitrogen-hydrogen bonds can interact with other hydrogen bond acceptors, forming intermolecular interactions that influence its physical and chemical properties.
- Solubility: Urea is highly soluble in water. The polar nature and hydrogen bonding ability of urea enable it to dissolve easily in polar solvents like water.
- Amide Bond: The carbon-nitrogen bond in urea is an amide bond, which is a common functional group in proteins and other biological molecules. The amide bond contributes to the molecule’s stability and reactivity.
Applications of Urea
Urea finds extensive applications in various industries, including:
- Fertilizer: Urea is one of the most widely used nitrogen fertilizers. It provides plants with essential nitrogen for growth and development.
- Chemical Synthesis: Urea is a starting material for the synthesis of a wide range of chemicals, including plastics, resins, and adhesives.
- Pharmaceuticals: Urea is used as a component in several pharmaceutical formulations, such as creams and ointments, due to its moisturizing and exfoliating properties.
- Wastewater Treatment: Urea is employed in wastewater treatment plants to enhance biological nutrient removal processes.
The global urea market is estimated to reach $131.5 billion by 2026, according to Allied Market Research. Its increasing demand is attributed to growing population and urbanization, which drive the need for fertilizers and chemical products.
Tips and Tricks for Working with Urea
- Handling Precautions: Urea is a non-toxic substance, but it can irritate the eyes and skin. Proper protective gear should be worn when handling urea.
- Storage Conditions: Urea should be stored in a cool, dry place away from direct sunlight. Exposure to moisture can cause caking or lumping.
- Chemical Reactivity: Urea reacts with strong acids and bases. It can also decompose under high temperatures, releasing ammonia and carbon dioxide.
- Environmental Considerations: Urea can contribute to eutrophication if excessive amounts enter water bodies. Proper disposal practices should be followed.
Conclusion
Urea’s Lewis structure provides a fundamental understanding of its chemical bonding and properties. Its polarity, hydrogen bonding, and amide bond contribute to its diverse applications in industries such as agriculture, chemical synthesis, pharmaceuticals, and wastewater treatment. By understanding the urea Lewis structure, researchers and scientists can continue to explore new applications for this versatile compound.