Introduction

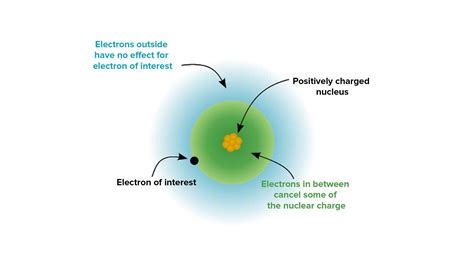
The concept of effective nuclear charge (ENC) plays a pivotal role in understanding the behavior and properties of chemical elements. In this article, we delve into the fascinating world of oxygen’s ENC, exploring its significance, variations, and practical applications.
Defining Effective Nuclear Charge
Effective nuclear charge refers to the positive charge experienced by an electron in an atom or ion. It is influenced by the number of protons in the nucleus and the screening effect of intervening electrons. The ENC is crucial for determining an electron’s energy levels, ionization potential, and chemical reactivity.
Determining the ENC of Oxygen
In the ground state of oxygen, there are eight electrons distributed in energy shells as follows:
- 1s2
- 2s2
- 2p4
The nuclear charge (Z) of oxygen is +8. However, the inner electrons (1s and 2s) effectively shield the outer electrons (2p) from the full nuclear charge. As a result, the ENC (Z’) experienced by the outermost 2p electrons is lower than +8:
Z' = Z - Σ(n - 1)
where:
- Z is the atomic number
- Σ(n – 1) is the sum of the principal quantum numbers of the inner electrons
In oxygen’s case, the ENC for the 2p electrons is +3. This means that the outermost electrons experience an attraction to the nucleus as if it had a charge of +3.
Variations in ENC
The ENC of oxygen can vary depending on the oxidation state of the atom. For example, in the O2- ion, the extra electron occupies a 2p orbital, increasing the screening effect and reducing the ENC experienced by the other 2p electrons. Consequently, the ENC of the 2p electrons in O2- is only +2.
Significance of ENC
The ENC has far-reaching implications for the properties of oxygen:
- Ionization Potential: Higher ENC increases the ionization potential, making it more difficult to remove electrons.
- Electronegativity: High ENC enhances electronegativity, leading to a tendency to attract electrons from other atoms.
- Bonding Ability: ENC influences the type and strength of chemical bonds formed. Higher ENC leads to stronger polar bonds.
Applications of ENC
The understanding of ENC finds practical applications in diverse fields:
- Industrial Chemistry: Controlling the ENC of oxygen-containing molecules improves catalytic processes and the synthesis of pharmaceuticals.
- Materials Science: Tailoring the ENC of oxygen atoms in semiconductors can enhance their electrical and optical properties.
- Environmental Chemistry: Understanding the ENC of oxygen in pollutants helps determine their reactivity and potential toxicity.
Strategies to Increase ENC
Several strategies can increase the ENC of oxygen:
- Increasing Oxidation State: A higher oxidation state leads to a lower number of valence electrons and, consequently, less electron screening.
- Ligand Binding: Coordinating oxygen atoms to metal ions with high charges can effectively increase the ENC by reducing the electron density around the oxygen.
- Electron-Withdrawing Substituents: Introducing electron-withdrawing groups adjacent to oxygen atoms can lower the electron density and increase the ENC.
Effective Applications of ENC
- Battery Technology: The ENC of oxygen in electrode materials plays a crucial role in determining battery capacity and efficiency.
- Catalysis: Modifying the ENC of oxygen-containing catalysts enhances their activity and selectivity in reactions such as hydrogen production and air pollution control.
- Drug Development: Understanding the ENC of oxygen in bioactive molecules aids in the design of drugs with improved efficacy and reduced side effects.
Conclusion
The effective nuclear charge of oxygen is a fundamental property that governs its behavior and properties. By understanding the variations and significance of ENC, researchers and scientists can harness its potential for numerous applications in industry, science, and medicine. In this comprehensive guide, we have provided an in-depth exploration of oxygen’s ENC, empowering readers with the knowledge to unlock its full potential and drive innovation.
Questions for Customer Engagement
- How does the ENC of oxygen impact the ionization potential of compounds?
- Can you identify specific examples where understanding ENC has led to practical advancements?
- How might the manipulation of ENC in oxygen-containing materials revolutionize future technologies?
Comparison of Strategies to Increase ENC
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Increasing Oxidation State | Strongest increase in ENC | May alter other properties |
| Ligand Binding | Moderate increase in ENC | Requires careful selection of ligands |
| Electron-Withdrawing Substituents | Weaker increase in ENC | Can introduce steric hindrance |
Tables
| Table 1: Variation of ENC in Oxygen Species |
|—|—|
| Species | ENC (2p Electrons) |
| O | +3 |
| O2- | +2 |
| H2O | +2.67 |
| CO2 | +3.33 |
| Table 2: Applications of ENC in Industry |
|—|—|
| Application | Impact of ENC |
| Catalytic Converters | Reduced emissions |
| Polymer Synthesis | Improved properties |
| Fuel Cells | Enhanced efficiency |
| Table 3: Strategies to Increase ENC in Oxygen |
|—|—|
| Strategy | Mechanism |
| Increasing Oxidation State | Reduction of electron shielding |
| Ligand Binding | Electron withdrawal from oxygen |
| Electron-Withdrawing Substituents | Destabilization of lone pairs |
| Table 4: Examples of Effective Applications of ENC |
|—|—|
| Application | ENC Impact | Benefit |
| Lithium-Ion Batteries | Optimizing oxygen redox reactions | Increased capacity |
| Solar Cells | Enhancing light absorption | Higher efficiency |
| Cancer Treatment | Targeting oxygen consumption | Improved efficacy |