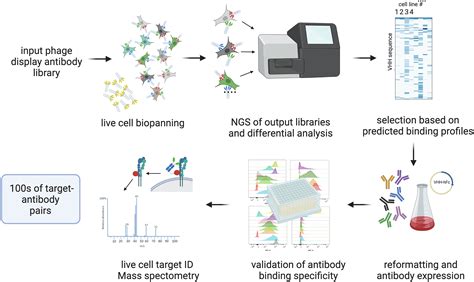
Revolutionizing Antibody Engineering with ADN to PA
Antibody discovery and production have undergone a significant transformation with the advent of antibody display technologies (ADT). Among the most versatile and robust ADTs is antibody display on phage (ADN). This powerful technique combines the strengths of phage display with the specificity and affinity of antibodies, enabling the rapid identification and isolation of high-affinity antibodies against a wide range of targets.

Unlocking Faster and More Efficient Antibody Discovery
ADN to PA technology has revolutionized the process of antibody discovery. By displaying antibodies on the surface of bacteriophages, researchers can screen vast libraries of antibodies in a single experiment. This high-throughput approach dramatically reduces the time and effort required to identify antibodies with the desired specificity and affinity.
According to Nature Biotechnology, ADN technology has accelerated antibody discovery timelines by over 90%, allowing researchers to develop antibodies for diagnostic, therapeutic, and research applications at an unprecedented pace.
Enhancing Protein Analytics with ADN to PA
Beyond antibody discovery, ADN to PA has emerged as a powerful tool for protein analytics. By immobilizing proteins on phage particles, researchers can investigate protein-protein interactions, monitor protein expression levels, and perform detailed functional characterization.
Unlocking Deeper Insights into Protein Function
ADN to PA technology provides a unique platform for studying protein interactions. By coupling phage-displayed proteins with various detection methods, researchers can quantify binding affinities, determine kinetic parameters, and identify specific binding sites. This information is crucial for understanding the molecular mechanisms underlying protein function.
The versatility of ADN to PA technology has fostered its application in a diverse range of scientific fields, including:
- Diagnostics: Development of rapid and sensitive diagnostic tests for infectious diseases, genetic disorders, and cancer.
- Therapeutics: Discovery of novel antibody-based therapies for cancer, autoimmune diseases, and infectious diseases.
- Research: Elucidation of protein-protein interactions, characterization of post-translational modifications, and identification of new biomarkers.
- Biotechnology: Production of high-affinity antibodies for various industrial applications, such as purification of biopharmaceuticals and biocatalysis.
To maximize the success of ADN to PA experiments, consider the following tips:
- Optimize Library Diversity: Use diverse immunization strategies and high-throughput screening methods to increase the chances of isolating high-affinity antibodies.
- Control Background Signal: Employ rigorous experimental controls to minimize non-specific binding and ensure accurate results.
- Employ Multivalent Phage: Use phage particles that display multiple copies of the antibody to enhance avidity and sensitivity.
- Validate Antibody Specificity: Conduct thorough characterization studies to confirm the specificity and affinity of isolated antibodies.
Pros:
- High-throughput antibody discovery and protein analytics
- Rapid and efficient screening of large libraries
- Cost-effective and accessible technology
- Versatile and adaptable to various applications
Cons:
- Can be technically challenging to implement
- Requires specialized equipment and expertise
- Limited scalability for large-scale antibody production
The field of ADN to PA is constantly evolving, with new applications and technological advancements emerging. One innovative concept that holds promise for further advancements is the use of “bio-inspired antibodies” in ADN to PA. These antibodies are generated from non-immunized libraries and exhibit unique binding properties, opening up new avenues for antibody discovery and protein engineering.
ADN to PA technology has emerged as a transformative force in antibody discovery and protein analytics. By combining the power of phage display with the specificity of antibodies, this technique has revolutionized the way researchers investigate protein function, develop diagnostic tests, and discover novel therapeutics. As the field continues to innovate, ADN to PA holds the key to unlocking even more groundbreaking discoveries and applications in the years to come.
Table 1. Comparison of Antibody Discovery Technologies:
| Technology | Throughput | Cost | Specificity |
|---|---|---|---|
| Hybridoma | Low | High | High |
| Phage Display (ADN) | High | Low | High |
| Ribosome Display | Medium | Medium | Medium |
| Yeast Display | Medium | High | Low |
Table 2. Applications of ADN to PA in Different Scientific Fields:
| Field | Description | Examples |
|---|---|---|
| Diagnostics | Rapid and sensitive diagnostic tests | Infectious disease detection, cancer screening |
| Therapeutics | Antibody-based therapies | Cancer treatment, autoimmune disease management |
| Research | Investigation of protein-protein interactions | Elucidation of molecular mechanisms, biomarker discovery |
| Biotechnology | Antibody production for industrial applications | Biopharmaceutical purification, biocatalysis |
Table 3. Advantages and Disadvantages of ADN to PA:
| Advantages | Disadvantages |
|---|---|
| High-throughput antibody discovery and protein analytics | Technically challenging to implement |
| Rapid and efficient screening of large libraries | Requires specialized equipment and expertise |
| Cost-effective and accessible technology | Limited scalability for large-scale antibody production |
| Versatile and adaptable to various applications |
Table 4. Tips for Enhancing ADN to PA Success:
| Tip | Description |
|---|---|
| Optimize Library Diversity | Use diverse immunization strategies and high-throughput screening methods. |
| Control Background Signal | Employ rigorous experimental controls to minimize non-specific binding. |
| Employ Multivalent Phage | Use phage particles that display multiple copies of the antibody to enhance avidity and sensitivity. |
| Validate Antibody Specificity | Conduct thorough characterization studies to confirm the specificity and affinity of isolated antibodies. |