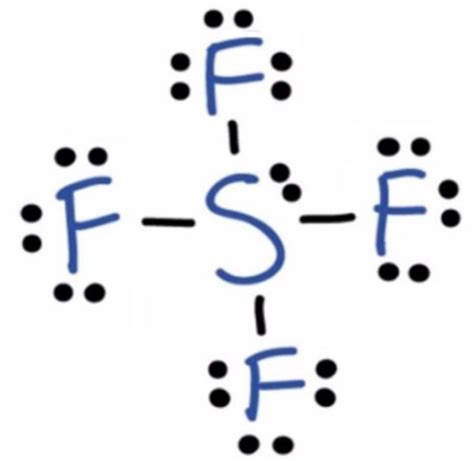
The Lewis dot structure of SF4 shows that the sulfur atom is surrounded by four fluorine atoms. Each fluorine atom has seven valence electrons, and the sulfur atom has six valence electrons. The Lewis dot structure of SF4 is:

F
|
S--F
|
F
The sulfur atom has a formal charge of 0, and each fluorine atom has a formal charge of -1.
Molecular Geometry and Bonding
SF4 has a see-saw molecular geometry. The sulfur atom is located at the center of the molecule, and the four fluorine atoms are located at the corners of a square. The bond angles between the sulfur atom and the fluorine atoms are 90 degrees.
The bonding in SF4 can be described using the valence shell electron pair repulsion (VSEPR) model. The VSEPR model predicts that the molecular geometry of a molecule is determined by the number of valence electron pairs around the central atom. In the case of SF4, the sulfur atom has six valence electrons, which form three bonding pairs and one lone pair. The three bonding pairs are arranged in a trigonal planar geometry, and the lone pair is located in one of the four corners of the square.
Physical and Chemical Properties
SF4 is a colorless gas with a boiling point of -38.4 degrees Celsius and a melting point of -120.4 degrees Celsius. It is soluble in water, and it reacts with water to form hydrogen fluoride and sulfur dioxide.
Applications
SF4 is used as an etching agent in the semiconductor industry. It is also used as a source of fluorine in the production of other chemicals.
Safety
SF4 is a toxic gas, and it can cause respiratory problems if inhaled. It is important to handle SF4 with care and to use proper safety equipment.
Further Reading
- Wikipedia: Sulfur tetrafluoride
- National Institute for Occupational Safety and Health: Sulfur Tetrafluoride
- Air Liquide: Sulfur Tetrafluoride
Frequently Asked Questions
What is the Lewis dot structure of SF4?
The Lewis dot structure of SF4 is:
F
|
S--F
|
F
What is the molecular geometry of SF4?
SF4 has a see-saw molecular geometry.
What are the physical and chemical properties of SF4?
SF4 is a colorless gas with a boiling point of -38.4 degrees Celsius and a melting point of -120.4 degrees Celsius. It is soluble in water, and it reacts with water to form hydrogen fluoride and sulfur dioxide.
What are the applications of SF4?
SF4 is used as an etching agent in the semiconductor industry. It is also used as a source of fluorine in the production of other chemicals.
What are the safety concerns associated with SF4?
SF4 is a toxic gas, and it can cause respiratory problems if inhaled. It is important to handle SF4 with care and to use proper safety equipment.