Introduction
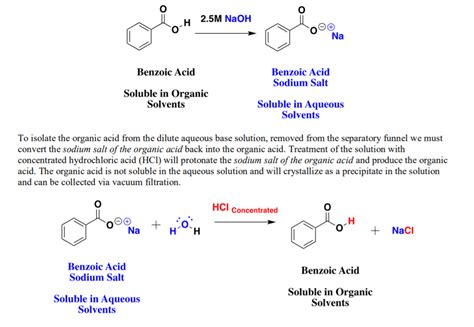
Benzoic acid, a widely used organic compound, reacts with sodium hydroxide (NaOH) to form sodium benzoate, a water-soluble salt. This reaction plays a vital role in various industries, including food preservation, pharmaceutical manufacturing, and chemical synthesis. In this article, we delve into the intricacies of this reaction, exploring its mechanism, applications, and implications.

Mechanism of the Reaction
The reaction between benzoic acid and NaOH is a classic acid-base reaction. Benzoic acid, a weak acid, donates a proton to NaOH, a strong base, resulting in the formation of sodium benzoate and water. The reaction can be represented as follows:
C6H5COOH + NaOH → C6H5COONa + H2O
This reaction is typically carried out in aqueous solutions, where the NaOH is dissolved in water. The rate of the reaction is influenced by several factors, including the concentration of reactants, temperature, and the presence of a catalyst.
Applications of the Reaction
The reaction of benzoic acid with NaOH has numerous applications, including:
- Food Preservation: Sodium benzoate is widely used as a food preservative due to its antimicrobial properties. It inhibits the growth of bacteria, yeast, and mold, thereby extending the shelf life of food products.
- Pharmaceutical Manufacturing: Sodium benzoate is employed in the preparation of various pharmaceutical formulations, such as cough syrups, antacids, and pain relievers. It acts as a buffer and preservative, stabilizing the active ingredients and preventing microbial contamination.
- Chemical Synthesis: Sodium benzoate is an intermediate in the synthesis of other organic compounds, including benzoyl chloride, benzyl alcohol, and benzaldehyde. These compounds find applications in the production of fragrances, dyes, and pharmaceuticals.
Implications of the Reaction
The reaction between benzoic acid and NaOH has several implications, including:
- Salt Formation: The reaction results in the formation of sodium benzoate, a water-soluble salt. This salt is readily soluble in aqueous solutions, facilitating its transport and utilization in various applications.
- pH Control: NaOH is a strong base, which can alter the pH of the solution. By controlling the amount of NaOH added, the pH can be adjusted to the desired level for specific applications.
- Buffering Capacity: Sodium benzoate acts as a buffer, resisting changes in pH upon the addition of small amounts of acid or base. This buffering capacity is essential in maintaining a stable pH environment in biological systems and industrial processes.
Novel Applications of the Reaction
The reaction between benzoic acid and NaOH holds potential for novel applications, including:
- Biodegradation Enhancements: Sodium benzoate has been shown to enhance the biodegradation of certain organic pollutants in soil and water. This could lead to the development of innovative waste treatment technologies.
- Biocatalysis: The use of enzymes to catalyze the reaction between benzoic acid and NaOH could offer green and sustainable alternatives to traditional chemical synthesis methods.
- Sensing and Diagnostics: The reaction between benzoic acid and NaOH can be used as the basis for chemical sensors and diagnostic tests. By monitoring the change in pH or the formation of sodium benzoate, it is possible to detect the presence of specific analytes.
Conclusion
The reaction between benzoic acid and NaOH is a versatile and important chemical process with wide-ranging applications. Understanding the mechanism, applications, and implications of this reaction is crucial for harnessing its potential in various fields. By exploring novel applications, we can unlock new possibilities and contribute to the advancement of science and technology.