Introduction
The reaction between calcium carbonate (CaCO3) and hydrochloric acid (HCl) is a fundamental chemical process with numerous applications in various fields. Understanding the mechanism, factors, and applications of this reaction is crucial for scientific research and industrial processes. This comprehensive article delves into every aspect of the CaCO3 and HCl reaction, providing a thorough understanding of its principles and practical implications.

The Reaction Mechanism
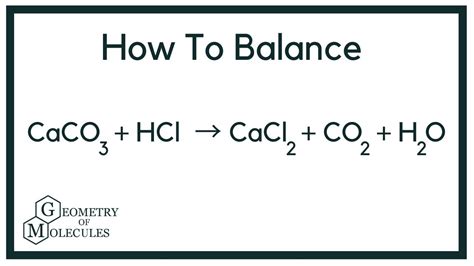
The reaction between CaCO3 and HCl can be represented by the following chemical equation:
CaCO3 + 2HCl → CaCl2 + H2O + CO2
In this reaction, calcium carbonate reacts with hydrochloric acid to form calcium chloride (CaCl2), water (H2O), and carbon dioxide (CO2). The reaction proceeds in two steps:
- Formation of Calcium Chloride: In the first step, HCl donates two protons (H+) to CaCO3, resulting in the formation of calcium chloride (CaCl2). This reaction releases water (H2O) as a byproduct.
- Release of Carbon Dioxide: In the second step, the remaining carbonate ion (CO32-) reacts with the protons from HCl to form carbonic acid (H2CO3). Carbonic acid is highly unstable and immediately decomposes into water (H2O) and carbon dioxide (CO2).
Factors Affecting the Reaction Rate
The rate of the reaction between CaCO3 and HCl is influenced by several factors, including:
- Concentration of HCl: The rate of the reaction increases with increasing concentration of HCl. This is because a higher concentration of HCl provides more protons for the reaction to proceed.
- Temperature: The rate of the reaction increases with increasing temperature. Elevated temperatures provide more energy for the reactants to overcome the activation energy barrier and react.
- Surface Area of CaCO3: The rate of the reaction increases with increasing surface area of CaCO3. A larger surface area provides more contact points between CaCO3 and HCl, facilitating the reaction.
- Agitation: Agitation, such as stirring or shaking, increases the rate of the reaction by enhancing the mixing of the reactants and increasing the collision frequency between CaCO3 and HCl molecules.
Applications of the CaCO3 and HCl Reaction
The reaction between CaCO3 and HCl has numerous practical applications in various fields:
- Antacid Production: Calcium carbonate is widely used as an antacid to neutralize stomach acid. It reacts with HCl in the stomach to produce calcium chloride, water, and carbon dioxide, which helps alleviate heartburn and indigestion.
- Soil Treatment: CaCO3 is used as a soil amendment to neutralize acidic soils. It raises the soil pH by reacting with HCl in the soil, making it more suitable for plant growth.
- Effluent Treatment: Calcium carbonate is used to treat wastewater effluents that contain high levels of HCl. The reaction neutralizes the acid and removes chloride ions, reducing the environmental impact of the effluent.
- Acid Rain Mitigation: CaCO3 is employed in acid rain mitigation strategies. It is spread over areas affected by acid rain to neutralize the HCl in the rainwater, reducing its corrosive effects on buildings and vegetation.
Common Mistakes to Avoid
When performing the CaCO3 and HCl reaction, it is important to avoid the following common mistakes:
- Using Impure Reagents: Impurities in CaCO3 or HCl can interfere with the reaction. It is essential to use pure reagents to ensure accurate results.
- Overheating the Reaction: Excessive heating can lead to the decomposition of CaCl2 and the formation of calcium oxide (CaO). This can alter the reaction stoichiometry and affect the desired outcome.
- Not Agitating the Reaction Mixture: Insufficient agitation can slow down the reaction rate. Vigorous stirring or shaking is recommended to enhance the mixing of the reactants.
- Using Excess HCl: Using excess HCl can result in the formation of acidic byproducts. It is important to use the stoichiometrically correct amount of HCl to avoid this issue.
Frequently Asked Questions (FAQs)
-
What is the role of carbon dioxide in the reaction?
Carbon dioxide is a byproduct of the reaction and does not participate in the main chemical process. It is released as a gas and does not affect the reaction stoichiometry. -
Why is it important to use pure reagents?
Impurities in the reagents can interfere with the reaction and affect the accuracy of the results. Using pure reagents ensures that the reaction proceeds as expected and produces the desired products. -
How can the reaction rate be increased?
The reaction rate can be increased by increasing the concentration of HCl, temperature, surface area of CaCO3, or by agitating the reaction mixture. -
What are some safety precautions when handling CaCO3 and HCl?
CaCO3 is a mild irritant to the skin and eyes, while HCl is a corrosive acid. Wear appropriate personal protective equipment (PPE) such as gloves, goggles, and a lab coat when handling these chemicals. -
What are some environmental applications of the CaCO3 and HCl reaction?
The reaction is used in soil treatment to neutralize acidic soils, effluent treatment to remove HCl from wastewater, and acid rain mitigation to neutralize HCl in rainwater. -
Can the reaction be used to synthesize other chemicals?
The reaction can be used to synthesize calcium salts, such as calcium nitrate or calcium sulfate, by reacting the resulting calcium chloride with other acids.
Conclusion
The reaction between CaCO3 and HCl is a fundamental chemical process with diverse applications in various fields. Understanding the reaction mechanism, factors affecting the reaction rate, and practical applications is essential for researchers, scientists, and industrial professionals. By adhering to best practices and avoiding common mistakes, it is possible to harness the full potential of this reaction and achieve desired outcomes.