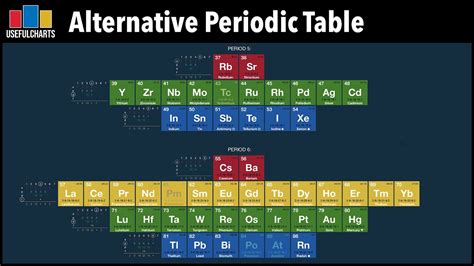
The periodic table, an indispensable tool for chemists and scientists alike, organizes the 118 known elements based on their atomic number, electron configuration, and chemical properties. Yet, for educational purposes or when exploring its fundamental nature, it can be insightful to examine a periodic table with no names.

Understanding the Elements by Their Properties
Stripping away the familiar element names unveils a tapestry of patterns and relationships that deepen our understanding of the elements.
- Atomic Number: The atomic number, represented by Z, is a unique identifier for each element. It indicates the number of protons in the nucleus and determines the number of electrons in a neutral atom.
- Group: Vertical columns in the table represent groups, which encompass elements with similar valence electron configurations. These electrons play a crucial role in chemical bonding and reactivity.
- Period: Horizontal rows in the table are called periods and indicate the number of electron shells in the atom. Elements within the same period have the same number of shells.
Exploring Patterns and Trends
By scrutinizing a periodic table with no names, we can discern several fundamental patterns:
1. Metallic and Nonmetallic Character: A diagonal line from top left to bottom right separates metals (to the left) from nonmetals (to the right). Metals are generally shiny, malleable, and good conductors of heat and electricity, while nonmetals are typically gas, solid, or liquid at room temperature and possess poor conductivity.
2. Reactivity: Reactivity increases down a group and decreases across a period. The most reactive elements are located at the bottom left of the table (e.g., potassium, sodium), while the least reactive elements reside at the top right (e.g., helium, neon).
3. Periodic Trends: As one traverses the periodic table from left to right or top to bottom, specific properties exhibit periodic trends. For instance, atomic radius, ionization energy, and electron affinity follow predictable patterns.
Applications in Education and Research
The periodic table with no names serves as a valuable educational tool, fostering the development of analytical and problem-solving skills. It challenges students to rely on the periodic table’s structure and patterns to deduce element properties and relationships.
Moreover, removing names facilitates the interrogation of the table itself as a scientific construct. Researchers can explore the relationships between different properties and develop models to explain the observed patterns and trends.
Common Mistakes to Avoid
When working with a periodic table with no names, it is imperative to avoid the following common mistakes:
- Confusing Periods and Groups: Periods indicate the number of electron shells, while groups represent valence electron configurations.
- Ignoring Element Z: It is essential to consider the atomic number of an element when deducing its properties and relationships.
- Oversimplifying Trends: While periodic trends generally hold true, there are exceptions and nuances that require careful consideration.
Pros and Cons of Using a Periodic Table with No Names
Pros:
- Facilitates pattern recognition and deepens understanding of element properties.
- Encourages analytical thinking and problem-solving.
- Opens avenues for exploring the periodic table’s structure and relationships.
Cons:
- May be more challenging for beginners or those unfamiliar with element names.
- Requires a higher level of conceptual understanding to navigate effectively.
- Can limit immediate access to specific element information.
Conclusion
The periodic table with no names offers a novel perspective on the fundamental building blocks of matter. By removing the familiarity of element names, it invites us to engage with the table’s structure, patterns, and relationships in a fresh and insightful way. Whether for educational purposes, scientific exploration, or simply broadening our understanding of the universe, a periodic table with no names is a valuable tool that empowers us to unlock a deeper appreciation for the elements that surround us.