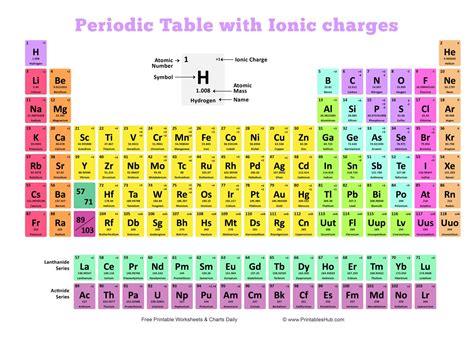
Introduction
The periodic table is a systematic arrangement of chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. Each element is assigned a unique symbol, atomic mass, and a set of charges that determine its chemical behavior. Understanding the charges of elements is crucial for comprehending their reactivity and interactions with other elements.

Atomic Structure and Charge
An atom consists of a positively charged nucleus, surrounded by negatively charged electrons. The nucleus contains protons and neutrons, which determine the atomic number and mass of the element. The number of electrons orbiting the nucleus is equal to the number of protons, resulting in a neutral overall charge. However, certain chemical reactions can cause an atom to gain or lose electrons, resulting in the formation of ions.
Types of Charges
-
Positive Charge (Cation): When an atom loses one or more electrons, it becomes positively charged. The resulting ion is called a cation. The charge of a cation is denoted with a superscript “+”. For example, sodium (Na) can lose one electron to form a sodium cation (Na+).
-
Negative Charge (Anion): When an atom gains one or more electrons, it becomes negatively charged. The resulting ion is called an anion. The charge of an anion is denoted with a superscript “-“. For example, chlorine (Cl) can gain one electron to form a chloride anion (Cl-).
-
Neutral Charge: In its natural state, an atom has an equal number of protons and electrons, resulting in a neutral overall charge.
Predicting Charges of Elements
The charges of elements can be predicted based on their position in the periodic table. Elements in Group 1 (alkali metals) typically lose one electron, forming cations with a charge of +1. Elements in Group 2 (alkaline earth metals) typically lose two electrons, forming cations with a charge of +2. Elements in Group 17 (halogens) typically gain one electron, forming anions with a charge of -1.
Consequences of Charges
The charges of elements have a profound impact on their chemical properties. Charged ions are attracted to each other by electrostatic forces, forming ionic bonds. For example, sodium cations and chloride anions can combine to form sodium chloride (NaCl). The charges of elements also influence their reactivity, solubility, and other physical and chemical properties.
Applications of Charges
Understanding the charges of elements is essential for numerous applications, including:
- Electrochemistry: The flow of electrons between charged ions is the basis of electrochemical cells, which are used in batteries and fuel cells.
- Industrial Chemistry: Electroplating and other industrial processes rely on the manipulation of charged ions to deposit or remove materials from surfaces.
- Medicine: The charged nature of ions is crucial for biological functions, such as nerve impulses and muscle contractions.
- Environmental Science: The charges of ions affect their behavior in the environment, influencing water quality, soil fertility, and atmospheric chemistry.
Periodic Table of Elements with Charges
The following table lists the charges of common elements, organized by group and period:
| Group | 1A (Alkali Metals) | 2A (Alkaline Earth Metals) | 3A | 4A | 5A | 6A | 7A (Halogens) | 8A (Noble Gases) |
|—|—|—|—|—|—|—|—|
| Period 1 | H+ | | | | | | F- | |
| Period 2 | Li+ | Be2+ | | | | | Cl- | |
| Period 3 | Na+ | Mg2+ | Al3+ | | | | Br- | |
| Period 4 | K+ | Ca2+ | | | | | I- | |
| Period 5 | Rb+ | Sr2+ | | | | | | |
| Period 6 | Cs+ | Ba2+ | | | | | | |
| Period 7 | Fr+ | Ra2+ | | | | | | |
Additional Resources
Conclusion
Understanding the charges of elements is essential for comprehending their chemical behavior, reactivity, and applications. The periodic table provides a systematic framework for organizing the charges of elements and predicting their properties. By mastering the concepts of charges, scientists and researchers can unlock a wealth of knowledge and develop innovative solutions across a wide range of fields.