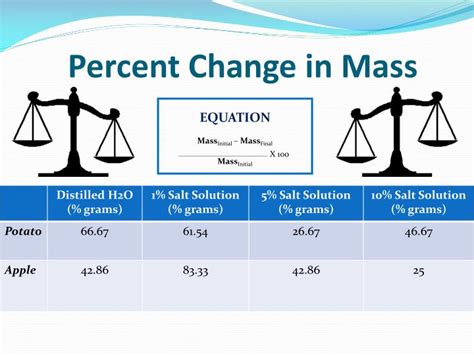
Introduction: Understanding Mass and Percent Change
Mass is a fundamental physical property of matter, representing the amount of material in an object. Quantifying mass is crucial in numerous scientific fields, from physics and chemistry to engineering and medicine. Percent change in mass refers to the proportional change in an object’s mass relative to its original mass. It is expressed as a percentage and serves as a valuable metric in various applications.

Formula for Calculating Percent Change in Mass
The formula for calculating percent change in mass is:
Percent Change in Mass = ((New Mass - Original Mass) / Original Mass) x 100%
Applications of Percent Change in Mass
Percent change in mass has a wide range of applications, including:
- Chemistry: Determining mass changes during chemical reactions to calculate reaction yield and stoichiometry.
- Physics: Measuring the mass lost by a rocket during propellant combustion or the mass of an object after a physical transformation.
- Medicine: Monitoring weight gain or loss in patients to assess nutritional status and metabolic health.
- Engineering: Evaluating the durability and performance of materials by measuring mass changes under various conditions.
Percent Change in Mass in Different Scenarios
Mass Gain
Mass gain occurs when an object increases in mass due to material addition or absorption. Some examples include:
- Chemical reactions: When reactants combine to form a heavier product.
- Biological growth: When organisms accumulate new cells and tissues.
- Absorption: When an object absorbs moisture or gases from its surroundings.
Mass Loss
Mass loss occurs when an object decreases in mass due to material removal or emission. Some examples include:
- Chemical reactions: When products are lighter than reactants, leading to mass loss.
- Combustion: When materials react with oxygen, breaking down into lighter components and releasing mass as gases.
- Evaporation: When liquids or solids convert into vapors and lose mass.
Tips and Tricks for Calculating Percent Change in Mass
- Ensure accurate measurements: Use calibrated scales or instruments to obtain precise mass readings.
- Consider significant figures: Report percent change in mass with an appropriate number of significant figures corresponding to the precision of the measurements.
- Handle negative values: If the new mass is less than the original mass, the percent change in mass will be negative, indicating a loss.
- Use a spreadsheet program: Utilize a spreadsheet application to automate calculations and minimize errors.
Tables for Reference
Table 1: Percent Change in Mass in Chemical Reactions
| Reaction | Original Mass | New Mass | Percent Change in Mass |
|---|---|---|---|
| Hydrogen + Oxygen -> Water | 2 g + 16 g | 18 g | 12.5% |
| Calcium Carbonate -> Calcium Oxide + Carbon Dioxide | 100 g | 56 g | -44% |
Table 2: Percent Change in Mass in Physical Transformations
| Transformation | Original Mass | New Mass | Percent Change in Mass |
|---|---|---|---|
| Ice Melting | 100 g | 100 mL | 0% |
| Combustion of Wood | 50 g | 25 g | -50% |
Table 3: Percent Change in Mass in Biological Processes
| Process | Original Mass | New Mass | Percent Change in Mass |
|---|---|---|---|
| Plant Growth | 100 g | 200 g | 100% |
| Weight Loss in Humans | 90 kg | 80 kg | -11.1% |
Table 4: Percent Change in Mass in Engineering Applications
| Application | Original Mass | New Mass | Percent Change in Mass |
|---|---|---|---|
| Corrosion of Metal | 100 g | 98 g | -2% |
| Tensile Testing of Materials | 50 g | 48 g | -4% |
Innovate: Exploring Novel Applications of Percent Change in Mass
Mass Sensors: Percent change in mass can be utilized in mass sensors to detect small changes in mass, enabling applications such as analytical chemistry and medical diagnostics.
Materials Characterization: Measuring mass changes under controlled conditions can provide insights into material properties, such as density, porosity, and mechanical strength.
Environmental Monitoring: Percent change in mass sensors can be employed to monitor air and water quality by detecting the presence of pollutants and contaminants.
Conclusion
Percent change in mass is a versatile concept with numerous applications in diverse fields. Its formula facilitates precise calculations, and its significance extends from chemical reactions to biological processes and engineering applications. By understanding percent change in mass, we gain a deeper understanding of material behavior and properties, enabling advancements in various scientific and technological domains.