Introduction

Organic chemistry, the study of carbon-based molecules, plays a pivotal role in countless aspects of our lives, from the medicines we take to the materials we use. To fully grasp the intricate world of organic molecules, it is essential to understand the concept of OCN- resonance structures. This article delves into the fascinating realm of resonance, exploring its implications for the structure and properties of organic molecules.
What are OCN- Resonance Structures?
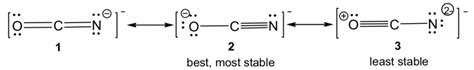
OCN- resonance structures are distinct but equivalent representations of a molecule that show the distribution of electrons within the molecule. They arise when there is a delocalized system of electrons, meaning the electrons are not confined to a single atom or bond but are spread over multiple atoms. This delocalization can occur when specific conditions, such as the presence of alternating double and single bonds or lone pairs of electrons, are met.
Delocalization and Resonance
In OCN- resonance structures, the delocalization of electrons leads to the formation of resonance hybrids, which are hypothetical structures that represent the actual molecular structure. These hybrids are not static but rapidly interconvert, resulting in a more stable molecular structure than any individual resonance structure. The extent of delocalization and the stability of the resonance hybrids are influenced by various factors, including the number of resonance structures possible, the electronegativity of the atoms involved, and the symmetry of the molecule.
Implications for Molecular Structure
OCN- resonance structures have profound implications for the structure and properties of organic molecules. By distributing the electron density over multiple atoms, resonance can lead to:
- Increased bond lengths: The delocalization of electrons weakens the bonds between atoms, resulting in longer bond lengths.
- Reduced reactivity: The delocalization of electrons makes the molecule less reactive towards electrophiles, as the positive charge is dispersed over multiple atoms.
- Enhanced stability: Resonance hybrids are typically more stable than any individual resonance structure, providing the molecule with increased stability.
Applications of Resonance Structures
The understanding of resonance structures is crucial in various fields, including:
- Chemistry: Predicting the structure, stability, and reactivity of organic molecules.
- Drug design: Designing drugs that interact effectively with specific biological targets.
- Materials science: Developing novel materials with tailored properties.
Case Studies
Benzene: Its molecular structure involves alternating double and single bonds. Resonance among these bonds results in the formation of a highly stable delocalized pi-electron system, contributing to its inertness and low reactivity.
Carboxylic acids: The carboxyl group contains a delocalized resonance structure, leading to enhanced acidity and the ability to form strong intermolecular hydrogen bonds.
Imidazole: This heterocyclic compound exhibits resonance between two equivalent structures, resulting in a highly aromatic system with enhanced stability. This property makes it an essential component in many biological molecules, including histidine and histamine.
Tables
| Property | Effect of Resonance |
|---|---|
| Bond Length | Increased |
| Reactivity | Decreased |
| Stability | Enhanced |
| Aromaticity | Increased |
| Molecule | Resonance Structures | Stability |
|---|---|---|
| Benzene | 2 | High |
| Carboxylic acid | 2 | Moderate |
| Imidazole | 2 | High |
| Application | Benefit |
|---|---|
| Drug design | Improved drug efficacy and selectivity |
| Materials science | Enhanced material properties |
| Organic synthesis | Prediction of reaction outcomes and design of synthetic strategies |
Conclusion
OCN- resonance structures are powerful tools for understanding the structure, properties, and reactivity of organic molecules. By recognizing the significance of delocalization and resonance, chemists can gain deep insights into the molecular world and pave the way for innovative applications in medicine, materials science, and beyond.