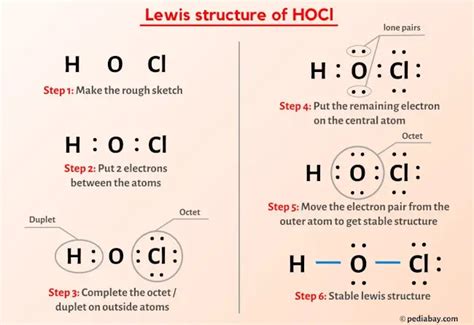
In the realm of chemistry, understanding the electronic structure of molecules is crucial for deciphering their properties and predicting their reactivity. One powerful tool employed for this purpose is the OCL (Orbital Counting Lewis) structure. This article delves into the intricacies of OCL Lewis structures, empowering readers with the knowledge to construct and interpret these fundamental representations of molecular bonding.

Embarking on the OCL Lewis Structure Odyssey
The OCL Lewis structure is a pictorial representation that depicts the arrangement of atoms and their valence electrons in a molecule. It provides a framework for comprehending the manner in which atoms share or transfer electrons to establish chemical bonds, forming the basis for molecular interactions.
Steps to Constructing an OCL Lewis Structure
- Determine the Total Number of Valence Electrons: Sum the valence electrons contributed by each atom in the molecule.
- Connect the Atoms: Connect the atoms with single bonds until each atom has a complete octet of electrons (except for hydrogen, which requires only two).
- Distribute the Remaining Electrons: Distribute the remaining valence electrons as lone pairs on the appropriate atoms, ensuring that no atom exceeds an octet.
- Check for Formal Charges: Calculate the formal charges of each atom to ensure that the structure is neutral or has the correct overall charge.
Delving into the Significance of OCL Lewis Structures
OCL Lewis structures serve as invaluable tools for chemists, providing insights into:
- Molecular Geometry: The arrangement of atoms in a molecule determines its shape and properties. OCL Lewis structures assist in predicting molecular geometry based on VSEPR (Valence Shell Electron Pair Repulsion) theory.
- Chemical Bonding: By revealing the distribution of electrons, OCL Lewis structures elucidate the nature of chemical bonds, whether they are covalent, ionic, or metallic.
- Reactivity: The electron arrangement in a molecule influences its reactivity and ability to undergo chemical reactions. OCL Lewis structures aid in predicting the behavior of molecules under specific conditions.
OCL Lewis Structure: A Journey of Evolution
Since its inception, the OCL Lewis structure has evolved to encompass various modifications and refinements. Among these advancements are:
- Resonance Structures: Many molecules possess multiple valid OCL Lewis structures known as resonance structures. These structures represent the delocalization of electrons, offering a more comprehensive picture of molecular bonding.
- Formal Charges: The concept of formal charges helps determine the stability of resonance structures and identify potential reaction sites within a molecule.
- Hybridization: The hybridization of atomic orbitals influences molecular geometry and bonding characteristics. OCL Lewis structures can be modified to reflect the hybridization of atoms.
Applications of OCL Lewis Structures: Unlocking a World of Possibilities
The versatility of OCL Lewis structures extends across a vast spectrum of scientific disciplines, including:
- Inorganic Chemistry: Understanding the bonding and structure of inorganic compounds is essential for materials science, catalysis, and energy storage. OCL Lewis structures provide a foundational framework for comprehending these complex systems.
- Organic Chemistry: The intricate world of organic molecules relies heavily on OCL Lewis structures. They enable researchers to predict reactivity, design new compounds, and develop pharmaceuticals.
- Biochemistry: OCL Lewis structures are crucial for deciphering the intricate structures of biomolecules, such as proteins, DNA, and RNA. This knowledge underpins advancements in medical research, drug development, and biotechnology.
Common Mistakes to Avoid in OCL Lewis Structure Construction
- Ignoring Valence Electrons: Neglecting to consider all valence electrons can lead to incorrect structures and misinterpretations of bonding.
- Overcounting Electrons: Double-counting valence electrons results in an overestimation of the number of bonding pairs and lone pairs, distorting the molecular structure.
- Incomplete Octet: Failing to provide a complete octet of electrons for each atom, except for hydrogen, can compromise the accuracy of the OCL Lewis structure.
Conclusion: OCL Lewis Structures – The Pillars of Molecular Understanding
OCL Lewis structures stand as indispensable tools in the arsenal of chemists, offering a comprehensive understanding of molecular bonding and reactivity. By unraveling the intricate arrangement of electrons within molecules, OCL Lewis structures pave the way for predicting their properties, designing new materials, and elucidating the underpinnings of chemical reactions. Embracing the power of OCL Lewis structures empowers chemists and researchers to delve deeper into the fascinating realm of chemistry.