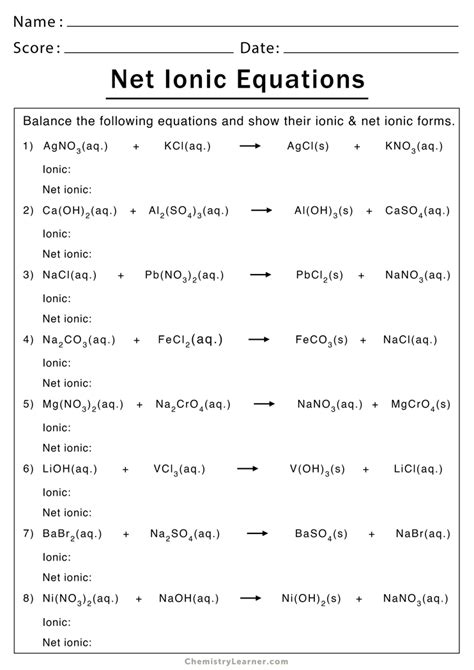
Net ionic equations simplify complex chemical reactions by representing only the essential ions involved in the reaction. They are vital for understanding the chemistry behind various chemical processes. This guide provides detailed practice problems and tips to help you master the concept of net ionic equations.

Understanding Net Ionic Equations
A net ionic equation is a chemical equation that represents the changes that occur when two or more ions interact. It eliminates spectator ions, which do not participate in the reaction. By focusing on the ions involved in the reaction, net ionic equations provide a clear picture of the chemical transformation.
For example, consider the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3):
NaCl + AgNO3 → NaNO3 + AgCl
The spectator ions (Na+ and NO3–) are present on both sides of the reaction, so they can be eliminated from the equation. The resulting net ionic equation is:
Ag+ + Cl- → AgCl
Practice Problems
- Write the net ionic equation for the reaction between sulfuric acid (H2SO4) and potassium hydroxide (KOH).
- Predict the products of a reaction between copper(II) sulfate (CuSO4) and barium chloride (BaCl2).
- Balance the following net ionic equation: MnO4– + H+ + Cl– → Mn2+ + Cl2 + H2O.
- Identify the spectator ions in the reaction between calcium chloride (CaCl2) and sodium phosphate (Na3PO4).
- Write a net ionic equation for a reaction that produces water.
Tips and Tricks
- Remember that spectator ions do not participate in the reaction, so they can be ignored in net ionic equations.
- Identify the ions present in the reactants and products, and determine which ones participate in the reaction.
- Balance the charges on both sides of the equation to ensure that it is chemically valid.
- Practice writing net ionic equations for a variety of reactions to build your understanding.
Step-by-Step Approach
- Write the molecular equation.
- Identify the spectator ions.
- Eliminate the spectator ions.
- Balance the charges on both sides of the equation.
- Write the balanced net ionic equation.
Applications of Net Ionic Equations
Net ionic equations have numerous applications in various fields, including:
- Analytical Chemistry: Identifying and quantifying ions in solutions.
- Electrochemistry: Predicting the products of electrolysis reactions.
- Environmental Chemistry: Understanding chemical reactions in environmental systems.
- Industrial Chemistry: Optimizing chemical processes for desired products.
FAQs
-
What are the benefits of using net ionic equations?
– They simplify chemical reactions by focusing on the essential ions involved.
– They provide a clear understanding of chemical transformations. -
How can I improve my ability to write net ionic equations?
– Practice regularly by solving problems and reviewing concepts.
– Refer to tables and resources that provide information on common ions and their reactions. -
What are some common misconceptions about net ionic equations?
– Spectator ions do participate in the reaction. (They do not)
– Net ionic equations can be written for all chemical reactions. (Not all)
– Net ionic equations always have a 1:1 mole ratio of ions. (Not always) -
What is a spectator ion?
– A spectator ion is an ion that does not participate in a chemical reaction and appears on both sides of the equation. -
What is the purpose of balancing charges in a net ionic equation?
– Balancing charges ensures that the overall charge on both sides of the equation is zero, making the equation chemically valid. -
How can net ionic equations be used to predict reaction products?
– By identifying the ions that participate in the reaction and using stoichiometry, net ionic equations can help predict the products formed.
Tables for Reference
Table 1: Common Ions and Their Spectator Status
| Ion | Spectator |
|---|---|
| Na+ | Yes |
| K+ | Yes |
| Cl– | No |
| NO3– | Yes |
| SO42- | No |
| CO32- | No |
Table 2: Solubility Rules for Common Compounds
| Compound | Solubility |
|---|---|
| NaCl | Soluble |
| AgCl | Insoluble |
| BaSO4 | Insoluble |
| CaCO3 | Insoluble |
| Mg(OH)2 | Insoluble |
Table 3: Acid-Base Neutralization Reactions
| Acid | Base | Products |
|---|---|---|
| HCl | NaOH | NaCl + H2O |
| H2SO4 | KOH | K2SO4 + H2O |
| HNO3 | NH3 | NH4NO3 |
Table 4: Precipitation Reactions
| Reactants | Products |
|---|---|
| AgNO3 + NaCl | AgCl + NaNO3 |
| CuSO4 + BaCl2 | BaSO4 + CuCl2 |
| CaCl2 + Na2CO3 | CaCO3 + NaCl |