Introduction

Sodium hydroxide (NaOH), also known as lye or caustic soda, is a versatile chemical compound with wide-ranging industrial and household applications. Its unique properties, which stem from its molecular structure, make it an indispensable material in various industries. This article provides a comprehensive overview of the NaOH Lewis dot structure, shedding light on its electron configuration, bonding characteristics, and the implications for its chemical reactivity.
Understanding the NaOH Lewis Dot Structure
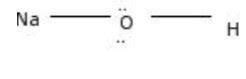
A Lewis dot structure is a graphical representation of a molecule’s electron distribution. It provides insights into the molecule’s bonding and molecular geometry. The NaOH Lewis dot structure is as follows:
O:Na:H
In this structure, the sodium (Na) atom is represented by a single dot, indicating its valence electron. The oxygen (O) atom is represented by six dots, representing its six valence electrons. The lone pair of electrons on the oxygen atom is represented by the two vertical dots, while the single electron on the hydrogen (H) atom is represented by a single dot.
Key Features of the NaOH Lewis Dot Structure
- Octet Rule: Both the oxygen and hydrogen atoms have eight electrons in their valence shells, satisfying the octet rule. This stable electron configuration makes NaOH a non-reactive species.
- Ionic Bond Formation: The sodium atom donates its single valence electron to the oxygen atom, resulting in the formation of an ionic bond between Na+ and OH-. This ionic bond accounts for NaOH’s strong basicity.
- Polar Covalent Bond: The O-H bond in NaOH is a polar covalent bond due to the difference in electronegativity between oxygen and hydrogen. The oxygen atom, being more electronegative, attracts the electrons towards itself, creating a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atom.
- Molecular Geometry: The NaOH molecule has a bent or V-shaped geometry due to the repulsion between the lone pair of electrons on the oxygen atom and the bonding pairs of electrons.
Applications of NaOH as a Result of its Lewis Dot Structure
The unique properties of NaOH, stemming from its Lewis dot structure, make it a highly versatile chemical with numerous applications across industries:
- Papermaking: NaOH is used in the production of paper by dissolving lignin, the glue-like substance that holds wood fibers together.
- Soap and Detergents: NaOH is used as a saponification agent in the production of soaps and detergents, which are essential for cleaning.
- Textile Manufacturing: NaOH is used in the dyeing and bleaching of textiles, enhancing their appearance and durability.
- Food Processing: NaOH is used in the processing of various foods, such as olives and pretzels, to soften and preserve them.
- Water Treatment: NaOH is used in water treatment plants to neutralize acidic wastewater and adjust pH levels.
Strategies for Enhancing the Applications of NaOH
Researchers are actively exploring new and innovative ways to leverage the properties of NaOH:
- Nanotechnology: Incorporating NaOH into nanomaterials could lead to novel applications in electronics, medicine, and energy.
- Advanced Materials: Modifying NaOH with other chemicals or materials could produce composites with enhanced strength, durability, and functionality.
- Environmental Remediation: NaOH can be used in innovative approaches to treat environmental pollutants and restore contaminated ecosystems.
FAQs
- What is the molecular weight of NaOH? 40.00 g/mol
- What is the melting point of NaOH? 318 °C (604 °F)
- What is the boiling point of NaOH? 1388 °C (2530 °F)
- Is NaOH flammable? No
- Is NaOH toxic? Yes, NaOH is corrosive and can cause skin irritation, eye damage, and respiratory problems.
- How should NaOH be stored? NaOH should be stored in airtight containers in a cool, dry place away from incompatible materials.
Conclusion
The NaOH Lewis dot structure provides a valuable framework for understanding the molecular structure, bonding properties, and chemical reactivity of NaOH. By harnessing the unique characteristics of this compound, industries across the board utilize NaOH in diverse applications. Ongoing research efforts strive to uncover new and innovative uses for NaOH, expanding its versatility and impact in various fields.