N2H4, also known as hydrazine, is a colorless, flammable liquid with a pungent ammonia-like odor. It is used as a rocket propellant, in the production of plastics and pharmaceuticals, and as a reducing agent in various chemical reactions. The molecular shape of N2H4 is determined by its electronic structure and plays a crucial role in its physical and chemical properties.

Electronic Structure and Bonding
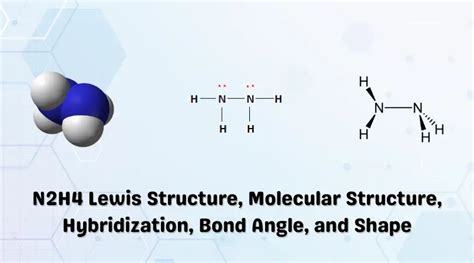
The N2H4 molecule consists of two nitrogen atoms and four hydrogen atoms. Each nitrogen atom has three valence electrons, which form three covalent bonds with the hydrogen atoms. The molecular formula of N2H4 can be written as H2N-NH2, indicating that the nitrogen atoms are connected by a single bond and each nitrogen atom is bonded to two hydrogen atoms.
The electronic structure of N2H4 can be described using molecular orbital theory. The valence electrons of the nitrogen and hydrogen atoms combine to form a set of molecular orbitals, which are orbitals that extend over the entire molecule. The lowest energy molecular orbital is the bonding sigma orbital (σ), which is formed by the overlap of the 1s orbitals of the two hydrogen atoms with the 2p orbitals of the nitrogen atoms. The next highest energy molecular orbital is also a bonding sigma orbital (σ), which is formed by the overlap of the 1s orbitals of the other two hydrogen atoms with the 2p orbitals of the nitrogen atoms.
The third and fourth molecular orbitals are non-bonding orbitals, which are formed by the overlap of the 2p orbitals of the nitrogen atoms with each other. The fifth and sixth molecular orbitals are antibonding orbitals, which are formed by the out-of-phase overlap of the 2p orbitals of the nitrogen atoms with the 1s orbitals of the hydrogen atoms.
Molecular Shape
The molecular shape of N2H4 is determined by the number and arrangement of its bonding and non-bonding electron pairs. In N2H4, there are two bonding electron pairs and two non-bonding electron pairs. The bonding electron pairs are arranged in a tetrahedral shape around each nitrogen atom, resulting in a bent molecular shape. The non-bonding electron pairs are located in the equatorial plane of the molecule, perpendicular to the N-N bond.
The bent molecular shape of N2H4 has several important implications. First, it prevents the molecule from rotating freely around the N-N bond. Second, it creates a dipole moment, making the molecule polar. Third, it affects the molecule’s reactivity, as the non-bonding electron pairs can participate in chemical reactions.
Physical and Chemical Properties
The molecular shape of N2H4 has a significant impact on its physical and chemical properties. The bent molecular shape prevents the molecule from packing closely together, resulting in a relatively low density (1.00 g/cm3). The polar nature of the molecule makes it soluble in water and other polar solvents. N2H4 is a reactive molecule, and it can undergo a variety of chemical reactions, including oxidation, reduction, and nucleophilic substitution.
Applications of N2H4
N2H4 is used in a variety of applications, including:
- Rocket propellant: N2H4 is used as a fuel for rocket engines. It is often used in combination with other fuels, such as hydrazine and hydrogen peroxide, to improve performance.
- Plastic production: N2H4 is used in the production of plastics, such as nylon and polyurethane. It is used as a blowing agent, which helps to create a porous structure in the plastic.
- Pharmaceuticals: N2H4 is used in the production of pharmaceuticals, such as isoniazid and hydralazine. It is used as a reducing agent, which helps to convert the starting materials into the desired products.
- Reducing agent: N2H4 is used as a reducing agent in various chemical reactions. It is often used in the reduction of metal ions, such as copper and iron.
Conclusion
The molecular shape of N2H4 is bent, which results in a variety of physical and chemical properties that make it useful for a variety of applications. N2H4 is used as a rocket propellant, in the production of plastics and pharmaceuticals, and as a reducing agent in various chemical reactions. The development of new applications for N2H4 is an ongoing area of research.