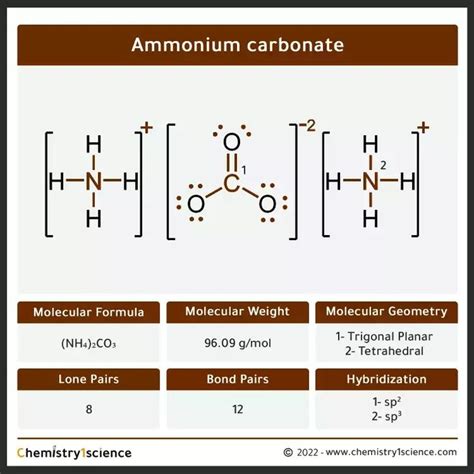
Unveiling the Molecular Mass of (NH₄)₂CO₃
Ammonium carbonate, a crucial inorganic salt, holds a significant presence in various scientific and industrial applications. Understanding its molecular mass is fundamental to comprehending its behavior and properties.

The molecular mass of (NH₄)₂CO₃, also known as ammonium bicarbonate, is calculated by adding the atomic masses of its constituent elements:
Molecular Mass of (NH₄)₂CO₃ =
2 × Atomic Mass of Nitrogen (N) +
8 × Atomic Mass of Hydrogen (H) +
1 × Atomic Mass of Carbon (C) +
3 × Atomic Mass of Oxygen (O)
= 2 × 14.01 amu + 8 × 1.01 amu + 1 × 12.01 amu + 3 × 16.00 amu
= 96.09 amu
Therefore, the molecular mass of ammonium carbonate is approximately 96.09 atomic mass units (amu).
Applications of Ammonium Carbonate
The remarkable properties of ammonium carbonate make it a versatile substance with a wide range of applications:
- Leavening agent in baking powder: Ammonium carbonate decomposes upon heating, releasing carbon dioxide that causes dough to rise. It is commonly used in baking powder and other leavening agents.
- Fertilizer: Ammonium carbonate serves as a valuable nitrogen source for agricultural purposes, contributing to plant growth and development.
- Fire retardant: The release of carbon dioxide and water vapor upon decomposition makes ammonium carbonate an effective flame retardant in fabrics and other flammable materials.
- Pharmaceutical industry: In pharmaceutical formulations, ammonium carbonate functions as an antacid to neutralize stomach acid. It also acts as a stimulant and expectorant in respiratory medications.
- Glass manufacturing: Ammonium carbonate is used in glassmaking to refine molten glass and improve its clarity.
Common Mistakes to Avoid
To accurately determine (NH₄)₂CO₃’s molecular mass, avoid these common mistakes:
- Using incorrect atomic masses: Ensure you use the most up-to-date atomic masses provided by authoritative sources like the International Union of Pure and Applied Chemistry (IUPAC).
- Miscounting the number of atoms: Carefully count the number of atoms of each element in the formula to avoid miscalculations.
- Converting units incorrectly: Ensure you convert the atomic mass units (amu) to grams per mole (g/mol) correctly using the appropriate conversion factor.
Frequently Asked Questions (FAQs)
1. What is the chemical structure of (NH₄)₂CO₃?
Answer: Ammonium carbonate consists of a central carbonate anion (CO₃²⁻) surrounded by two ammonium cations (NH₄⁺).
2. How does (NH₄)₂CO₃ decompose?
Answer: Upon decomposition, (NH₄)₂CO₃ releases carbon dioxide (CO₂), ammonia (NH₃), and water (H₂O).
3. Is (NH₄)₂CO₃ soluble in water?
Answer: Yes, ammonium carbonate is highly soluble in water, forming a colorless solution.
4. What are the safety precautions for handling (NH₄)₂CO₃?
Answer: Ammonium carbonate should be handled with caution due to its potential to release irritating gases. Use appropriate personal protective equipment (PPE) and avoid contact with skin and eyes.
5. How can I determine the percentage composition of (NH₄)₂CO₃?
Answer: Calculate the percentage composition by dividing the mass of each element in the molecule by the molecular mass and multiplying by 100%.
6. What are some innovative applications of (NH₄)₂CO₃?
Answer: Researchers are exploring the use of ammonium carbonate as a precursor for porous materials, catalysts, and flame retardants for electronic devices.
Conclusion
Understanding the molecular mass of (NH₄)₂CO₃ provides a crucial foundation for comprehending its chemical properties and practical applications. By understanding its composition and characteristics, scientists and engineers can harness its unique properties for various fields, including food processing, agriculture, pharmaceuticals, and advanced materials.