Introduction

Sulfur tetrachloride (SCl₄) is a versatile inorganic compound that finds widespread applications in various industries. Its molecular geometry plays a crucial role in determining its chemical properties, reactivity, and applications. This article delves into the molecular geometry of SCl₄, exploring its tetrahedral structure, key characteristics, and practical implications.
Tetrahedral Geometry: A Symmetrical Arrangement
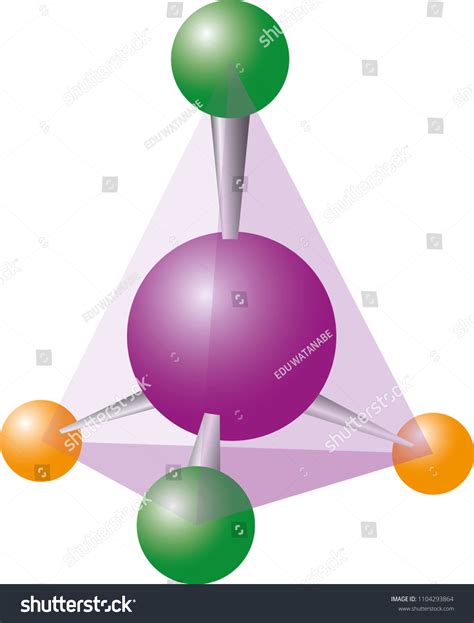
The molecular geometry of SCl₄ is tetrahedral, characterized by four chlorine atoms bonded to a central sulfur atom. The four Cl-S-Cl bond angles are approximately 109.5 degrees, resulting in a highly symmetrical structure. This tetrahedral geometry arises from the hybridization of the sulfur atom’s valence electrons into four equivalent sp³ hybrid orbitals.
Key Characteristics of Tetrahedral SCl₄
-
High Stability: The tetrahedral geometry of SCl₄ contributes to its high stability. The symmetrical arrangement of the chlorine atoms minimizes steric hindrance and electrostatic repulsions, resulting in a robust molecular structure.
-
Nonpolarity: SCl₄ is a nonpolar molecule due to the cancellation of individual bond polarities. The tetrahedral geometry ensures that the electron clouds around the sulfur atom are evenly distributed, resulting in no net dipole moment.
-
Low Reactivity: The tetrahedral geometry of SCl₄ hinders its reactivity compared to compounds with other geometries. The bulky chlorine atoms block access to the sulfur atom, making it less reactive towards nucleophiles and electrophilic species.
The tetrahedral geometry of SCl₄ underlies its unique properties and enables diverse applications:
-
Industrial Solvent: SCl₄ is widely used as a nonpolar solvent in the pharmaceutical and chemical industries. Its high stability and nonpolarity make it suitable for dissolving organic compounds, particularly those that are sensitive to polar solvents.
-
Chemical Intermediate: SCl₄ serves as a versatile intermediate in various chemical reactions. It is used to synthesize other sulfur-containing compounds, such as sulfur chlorides, thiosulfates, and sulfones.
-
Chlorinating Agent: SCl₄ is a strong chlorinating agent. It is employed in the production of chlorine-containing compounds, such as chlorinated hydrocarbons, dyes, and pharmaceuticals.
-
Insecticide and Herbicide: SCl₄ has been historically used as an insecticide and herbicide. However, its toxicity and environmental concerns have led to its restricted use in these applications.
To fully leverage the benefits of tetrahedral SCl₄, consider the following strategies:
-
Modify Surface Properties: Researchers are exploring the modification of SCl₄ surfaces to enhance its reactivity and selectivity. By introducing functional groups or altering the surface morphology, it is possible to tailor SCl₄ for specific applications.
-
Develop New Applications: The unique properties of tetrahedral SCl₄ can inspire the development of novel applications. For example, its nonpolarity and stability make it a promising candidate for use in advanced materials, such as polymer composites and optoelectronic devices.
-
Investigate Environmental Impact: The potential environmental risks associated with SCl₄ use must be carefully evaluated. By conducting thorough risk assessments and exploring mitigation strategies, researchers can minimize the adverse effects of SCl₄ on the environment.
Conclusion
The molecular geometry of SCl₄ is a remarkable example of how molecular structure dictates chemical behavior. The tetrahedral geometry of SCl₄ imparts stability, nonpolarity, and low reactivity, making it a valuable industrial solvent, chemical intermediate, and chlorinating agent. By understanding the molecular geometry of SCl₄ and exploring innovative strategies, scientists can harness its unique properties to develop new applications and address societal needs.