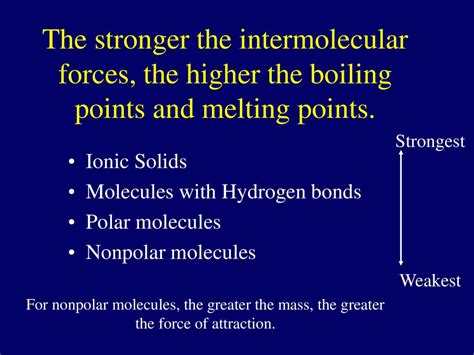
The melting point of a substance, the temperature at which it transitions from a solid to a liquid state, is a fundamental property that unravels intriguing insights into the intermolecular forces that govern its structure. Nonpolar molecules, characterized by the absence of permanent polarity or a net molecular dipole, exhibit unique melting point behaviors compared to their polar counterparts. Comprehending these distinctive characteristics provides a deeper understanding of the complex world of molecular interactions.

Understanding Nonpolar Molecules
Nonpolar molecules, composed of atoms with an equal sharing of electrons, possess minimal intermolecular forces. Their molecular structure lacks any permanent separation of charge, resulting in weak van der Waals forces that play a pivotal role in their behavior. Van der Waals forces encompass three distinct types:
- Dipole-dipole interactions: Interactions between polar molecules that possess permanent dipoles.
- London dispersion forces: Interactions that arise from the temporary fluctuations in electron distribution, creating instantaneous dipoles.
- Hydrogen bonding: Interactions that occur between molecules containing hydrogen atoms bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine.
Melting Point Trends in Nonpolar Molecules
The melting point of nonpolar molecules is predominantly influenced by the strength of van der Waals forces. Stronger van der Waals forces necessitate more energy input to overcome, leading to higher melting points. Factors affecting the strength of van der Waals forces include:
- Molecular size: Larger molecules with more surface area experience stronger van der Waals forces due to the increased number of contact points.
- Molecular shape: Molecules with compact, symmetrical shapes tend to have stronger van der Waals forces compared to elongated or branched structures.
- Polarizability: The ability of electrons within a molecule to be distorted can enhance van der Waals forces. Molecules with highly polarizable electron clouds exhibit stronger interactions.
Experimental Evidence and Applications
Numerous studies have quantified the melting points of various nonpolar molecules, providing valuable data for understanding their intermolecular forces. For instance, methane (CH₄), a small, symmetrical molecule with weak van der Waals forces, exhibits a melting point of -182.5 °C. Conversely, decane (C₁₀H₂₂), a larger, elongated molecule with stronger van der Waals forces, possesses a significantly higher melting point of -29.7 °C.
The distinct melting point characteristics of nonpolar molecules have led to their widespread use in various industrial and technological applications:
- Cryogenics: Nonpolar gases like nitrogen and helium, with extremely low melting points, are utilized as cryogens for cooling systems and cryopreservation.
- Lubricants: Nonpolar molecules, such as mineral oils and synthetic hydrocarbons, serve as lubricants due to their weak intermolecular forces, reducing friction and wear.
- Plastics: Nonpolar polymers, like polyethylene and polypropylene, exhibit high melting points and are widely used in the production of plastic materials.
- Surfactants: Nonpolar molecules with polar functional groups, known as surfactants, enable the emulsification and dispersion of immiscible liquids by reducing surface tension.
Tips and Tricks
- When analyzing melting point data, consider the purity of the substance as impurities can significantly alter the observed melting point.
- For accurate melting point determination, employ standardized techniques and calibrated equipment to ensure reliable measurements.
- Explore the use of differential scanning calorimetry (DSC) or thermogravimetric analysis (TGA) to obtain detailed information about melting behavior and thermal transitions.
Common Mistakes to Avoid
- Extrapolating trends: Avoid generalizing melting point trends based on limited data. Thorough analysis of multiple nonpolar molecules is essential for accurate conclusions.
- Oversimplifying intermolecular forces: Recognize that melting point is influenced by a combination of van der Waals forces, not just a single type.
- Ignoring crystal structure: Melting point can vary depending on the crystal structure of the nonpolar molecule. Consider the packing efficiency and molecular orientation within the crystal lattice.