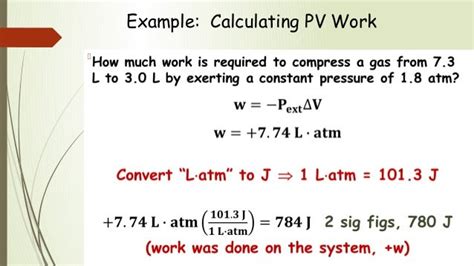
Understanding the Units
The liter (L) is a unit of volume in the metric system, while the atmosphere (atm) is a unit of pressure. The joule (J) is a unit of energy. The conversion from liter atm to joule is important for understanding the relationship between pressure, volume, and energy in various scientific and engineering applications.

Formula for Conversion
The formula for converting liter atm to joule is:
1 L atm = 101.325 J
This means that one liter of gas at atmospheric pressure contains 101.325 joules of energy.
Applications of Conversion
The conversion from liter atm to joule has numerous applications in different fields, including:
- Chemistry: Calculating the energy released or absorbed in chemical reactions.
- Physics: Determining the work done by gases in processes such as expansion or compression.
- Engineering: Designing systems that involve the use of gases under pressure, such as air compressors and engines.
Real-World Examples
Example 1: Tire Pressure
A car tire typically contains about 30 liters of air at a pressure of 2 atmospheres. Using the conversion formula, we can calculate the energy stored in the tire:
Energy = 30 L * 2 atm * 101.325 J/L atm
Energy = 6,079.5 J
Example 2: Gas Turbine Engine
A gas turbine engine uses the energy released by burning fuel to compress and expand gases. The energy released in the combustion process is typically measured in joules. If the engine burns 100 liters of fuel per hour at a pressure of 10 atmospheres, the energy released per hour can be calculated as:
Energy = 100 L * 10 atm * 101.325 J/L atm
Energy = 1,013,250 J/h
Table 1: Conversion Values
| Liters | Atmospheres | Joules |
|---|---|---|
| 1 | 1 | 101.325 |
| 10 | 1 | 1,013.25 |
| 100 | 1 | 10,132.5 |
| 1 | 2 | 202.65 |
| 1 | 3 | 303.975 |
| 1 | 4 | 405.3 |
Table 2: Applications in Different Fields
| Field | Application |
|---|---|
| Chemistry | Calculating reaction energies |
| Physics | Determining work done by gases |
| Engineering | Designing gas-based systems |
Table 3: Tips and Tricks
- Use unit conversions to ensure consistency in calculations.
- Pay attention to the units in the final result.
- Utilize online calculators or conversion tools for quick conversions.
Conclusion
The conversion from liter atm to joule is a fundamental concept that finds application in various scientific and engineering disciplines. By understanding the formula and its applications, professionals can accurately calculate the energy involved in gas-related processes.