Introduction

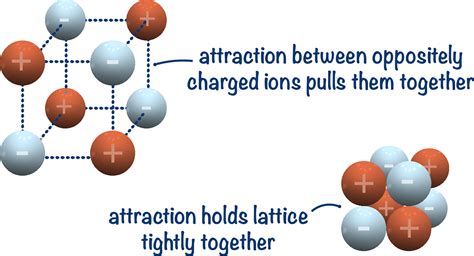
In the realm of chemistry, the cohesive forces that hold atoms or ions together in a crystal lattice are of paramount importance. These forces, known as lattice energy, govern the physical and chemical properties of ionic crystals. Among the various ionic compounds, lithium chloride (LiCl) stands out as a prime example for exploring the nature of lattice energy.
Lattice Energy of LiCl
Lattice energy refers to the energy required to separate all constituent ions in a crystal lattice into gaseous atoms. For LiCl, the lattice energy is the energy needed to dissociate one mole of solid LiCl crystals into one mole of gaseous Li+ and Cl- ions.
Factors Influencing LiCl Lattice Energy
Numerous factors contribute to the lattice energy of LiCl, including:
- Ionic Charge: The high charges of the Li+ (+1) and Cl- (-1) ions generate strong electrostatic attraction between them, resulting in a high lattice energy.
- Ionic Radii: The small ionic radii of Li+ (0.60 Å) and Cl- (1.81 Å) allow for close packing of ions within the lattice, maximizing electrostatic interactions.
- Crystal Structure: The face-centered cubic (fcc) crystal structure of LiCl facilitates efficient packing of ions, further enhancing lattice energy.
- Polarization: The polarizing power of Li+ ions induces a slight distortion of the electron cloud in Cl- ions, strengthening the electrostatic bond.
Measuring LiCl Lattice Energy
Various experimental techniques can be employed to determine the lattice energy of LiCl, including:
- Calorimetry: The dissolution of LiCl in water generates heat, which can be measured using calorimetry. The enthalpy change associated with this process is related to the lattice energy.
- Spectroscopy: The infrared and ultraviolet spectra of LiCl crystals provide information about the vibrational modes and electronic transitions within the lattice, which can be used to estimate lattice energy.
- Computational Methods: Quantum mechanical calculations, such as density functional theory (DFT), can simulate the interactions between ions in a crystal lattice and calculate lattice energy.
Applications of LiCl Lattice Energy
Understanding the lattice energy of LiCl has important implications for various applications:
- Electrolyte Design: The high lattice energy of LiCl makes it a suitable electrolyte for high-density batteries and energy storage devices.
- Solid-State Ionics: LiCl is used as a model material for studying ion transport in solid-state electrolytes, which is crucial for batteries and fuel cells.
- Materials Science: The fundamental knowledge of LiCl lattice energy aids in the design and development of new materials with enhanced properties.
Comparison with Other Ionic Compounds
The lattice energy of LiCl (853 kJ/mol) is significantly higher than that of other alkali halides due to the small ionic radii of Li+ and Cl-. Table 1 presents a comparison of lattice energies for selected ionic compounds.
| Ionic Compound | Lattice Energy (kJ/mol) |
|---|---|
| NaCl | 787 |
| KCl | 715 |
| RbCl | 677 |
| CsCl | 632 |
Novel Applications of LiCl Lattice Energy
Beyond traditional applications, the concept of lattice energy can be creatively extended to explore novel ideas:
- Lattice Energy as a Predictive Tool: The lattice energy of LiCl and other ionic compounds can serve as a predictor of solubility, melting point, and other physical properties.
- Ionic Lattice Architectures: By manipulating the lattice energy of LiCl, researchers can design crystals with specific shapes and properties, such as nano-octahedrons or porous structures.
- Energy Harvesting: The strong electrostatic forces within LiCl crystals can be harnessed to generate electricity through piezoelectric effects or other energy-conversion mechanisms.
Conclusion
The lattice energy of LiCl is a fundamental property that governs the cohesive forces within this ionic crystal. By understanding the factors influencing lattice energy and its applications, scientists and engineers can harness these forces to design and develop advanced materials and technologies. From high-density batteries to solid-state electrolytes, the knowledge of LiCl lattice energy continues to drive innovation in various fields.
References
- Atkins, P. W., & de Paula, J. (2014). Atkins’ inorganic chemistry (9th ed.). Oxford University Press.
- Haynes, W. M. (Ed.). (2016). CRC handbook of chemistry and physics (97th ed.). CRC Press.
- Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A: Crystal Physics, Diffraction, Theoretical and General Crystallography, 32(5), 751-767.