Introduction

The Lewis structure of HOBr, or hypobromous acid, is a graphical representation of the covalent bonding between its constituent atoms. It provides valuable insights into the molecule’s electronic configuration, molecular geometry, and chemical properties. This article will delve into the Lewis structure of HOBr, exploring its significance and providing a step-by-step guide to its construction.
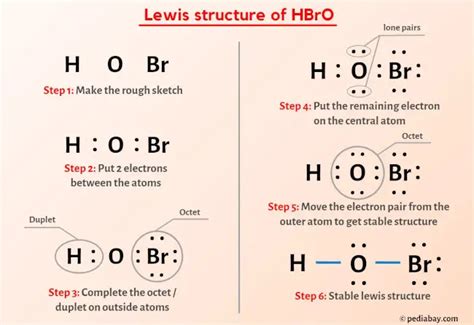
Drawing the Lewis Structure of HOBr
Step 1: Determine the Total Number of Valence Electrons
HOBr contains the following atoms and their respective valence electrons:
- H: 1
- O: 6
- Br: 7
Total valence electrons = 1 + 6 + 7 = 14
Step 2: Connect the Atoms with Single Bonds
Start by connecting the atoms with single bonds, resulting in the following structure:
H-O-Br
Step 3: Distribute Remaining Electrons as Lone Pairs
Distribute the remaining 10 valence electrons as lone pairs around the atoms, starting with the most electronegative element (oxygen).
H-O-Br
|
2
Step 4: Consider Multiple Bonds
Since the total valence electrons are not yet satisfied, explore the possibility of multiple bonds between the atoms. In this case, a double bond between oxygen and bromine is possible.
H-O=Br
|
2
Step 5: Satisfy Octet Rule
Ensure that all atoms, except hydrogen, have eight valence electrons (octet rule).
Final Lewis Structure:
H-O=Br
Significance of the Lewis Structure
The Lewis structure of HOBr reveals the following insights:
- Molecular Geometry: The electron-pair geometry around the bromine atom is trigonal pyramidal, but the molecular geometry is bent due to the lone pair on the oxygen atom.
- Bonding: There is a double bond between oxygen and bromine, represented by the two lines (=).
- Polarity: The polarity of the O-Br bond is due to the electronegativity difference between oxygen and bromine.
- Reactivity: The lone pair on the oxygen atom makes HOBr a good oxidizing agent.
Applications of HOBr
HOBr finds applications in various fields:
- Disinfectant: HOBr is a powerful disinfectant due to its oxidizing properties. It is used in water treatment, food processing, and healthcare settings.
- Bleaching Agent: HOBr is also used as a bleaching agent in the textile and paper industries.
- Organic Synthesis: HOBr participates in various organic reactions, including epoxidation, hydroxylation, and oxidative coupling.
- Biochemistry: HOBr plays a role in the immune system as a component of eosinophils, which are cells that fight infection and inflammation.
Conclusion
The Lewis structure of HOBr provides a fundamental understanding of its electronic configuration, molecular geometry, and chemical properties. This knowledge is essential for studying its reactivity and exploring its applications in disinfection, bleaching, organic synthesis, and biochemistry.
Additional Tables
Table 1: Key Properties of HOBr
| Property | Value |
|---|---|
| Molecular Weight | 92.91 g/mol |
| Boiling Point | 59°C |
| Density | 1.38 g/cm³ |
| Solubility in Water | Miscible |
| pKa | 8.2 |
Table 2: Applications of HOBr
| Application | Description |
|---|---|
| Disinfectant | Kills bacteria, viruses, and other microorganisms |
| Bleaching Agent | Whitens textiles and paper |
| Organic Synthesis | Participates in epoxidation, hydroxylation, and oxidative coupling reactions |
| Biochemistry | Component of eosinophils involved in immune defense |
Table 3: Lewis Structure of HOBr
| Atom | Valence Electrons |
|---|---|
| H | 1 |
| O | 6 |
| Br | 7 |
Table 4: Molecular Geometry of HOBr
| Shape | Bond Angle |
|---|---|
| Trigonal Pyramidal (around Br) | 109.5° |
| Bent (due to lone pair on O) |