Introduction
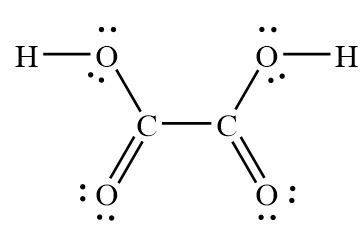
The Lewis structure of H2C2O4, commonly known as oxalic acid, is an essential representation of its molecular structure and bonding. Understanding the Lewis structure enables chemists to predict the properties and reactivity of this important compound.

Step-by-Step Approach to Drawing the Lewis Structure of H2C2O4
-
Count the total number of valence electrons:
– Carbon: 4 electrons per atom (2 atoms) = 8 electrons
– Hydrogen: 1 electron per atom (2 atoms) = 2 electrons
– Oxygen: 6 electrons per atom (4 atoms) = 24 electrons
– Total: 8 + 2 + 24 = 34 electrons -
Connect the atoms with single bonds:
– Connect the carbon atoms with a single bond to each other.
– Connect each carbon atom to two oxygen atoms with single bonds. -
Distribute the remaining electrons:
– Place double bonds between the carbon atoms and two of the oxygen atoms to satisfy the octet rule for carbon.
– Place lone pairs on the remaining two oxygen atoms to satisfy their octet rule. -
Check the octet rule:
– Each carbon atom has 8 electrons, each hydrogen atom has 2 electrons, and each oxygen atom has 8 electrons.
– The Lewis structure satisfies the octet rule for all atoms.
Resonance Structures of H2C2O4
The Lewis structure of H2C2O4 exhibits resonance, meaning that multiple resonance structures can be drawn for the molecule. Resonance structures are equivalent representations of the same molecule, differing only in the placement of double and single bonds. In the case of H2C2O4, there are two resonance structures:
O=C-COOH
|
O-C-O
O=C-O
| |
O=C-O
Resonance structures contribute to the stability of H2C2O4 by delocalizing the electrons and reducing the overall energy of the molecule.
Properties and Reactivity of H2C2O4
Oxalic acid (H2C2O4) is a colorless, crystalline solid with a sour taste. It is a diprotic acid, meaning that it can donate two protons (H+ ions). The dissociation constants are:
- Ka1 = 5.9 x 10^-2
- Ka2 = 5.6 x 10^-5
H2C2O4 is soluble in water, forming hydrogen oxalate ions (HC2O4-) and oxalate ions (C2O42-). It is also soluble in polar organic solvents like methanol and ethanol.
Oxalic acid is a reducing agent, meaning that it can donate electrons to other molecules or ions. This reactivity is due to the presence of the two carboxylic acid groups (-COOH).
H2C2O4 can form complexes with metal ions, such as calcium, magnesium, and iron. These complexes are often insoluble in water and can lead to kidney stones.
Applications of H2C2O4
Oxalic acid has a wide range of industrial, pharmaceutical, and domestic applications:
Industrial:
– Used as a bleaching agent in the textile industry
– Acts as a mordant in dyeing processes
– Used in the manufacture of leather, paper, and inks
Pharmaceutical:
– Used as an anticoagulant in certain medical procedures
– Can be found in some skin care products and cosmetics
Domestic:
– Used as a cleaning agent for removing rust and stains
– Can be found in some food products as an acidulant
Safety Considerations
Oxalic acid is a toxic substance and should be handled with care. It can cause irritation to the skin, eyes, and respiratory tract. Ingestion of oxalic acid can lead to kidney damage and even death.
It is important to follow proper safety precautions when working with H2C2O4, such as wearing protective gloves, eye protection, and a respirator.
Conclusion
The Lewis structure of H2C2O4 is a fundamental representation of its molecular structure and bonding. The molecule exhibits resonance, which contributes to its stability. H2C2O4 has a wide range of industrial, pharmaceutical, and domestic applications, but it is important to handle it with care due to its toxic nature.
Frequently Asked Questions (FAQs)
**Q: What is the hybridization of the carbon atoms in H2C2O4?**
A: The carbon atoms are sp2 hybridized.
**Q: Why does H2C2O4 exhibit resonance?**
A: H2C2O4 has resonance because the molecule can delocalize electrons through the carbon-oxygen double bonds, leading to multiple equivalent resonance structures.
**Q: What are some potential health risks associated with H2C2O4?**
A: Ingestion of oxalic acid can lead to kidney damage, hypocalcemia, and even death. It can also cause skin irritation and respiratory problems.
**Q: How can I safely dispose of H2C2O4?**
A: H2C2O4 should be disposed of according to local regulations. It is typically neutralized with a base and then disposed of as a solid waste.