Introduction
Aspirin, scientifically known as acetylsalicylic acid, is a widely used over-the-counter medication renowned for its analgesic, anti-inflammatory, and antipyretic properties. Composed of carbon, hydrogen, and oxygen atoms, aspirin’s molecular structure plays a critical role in its pharmacological effects. This article delves into the Lewis structure of aspirin, exploring its physical and chemical characteristics and highlighting its diverse applications in various fields.

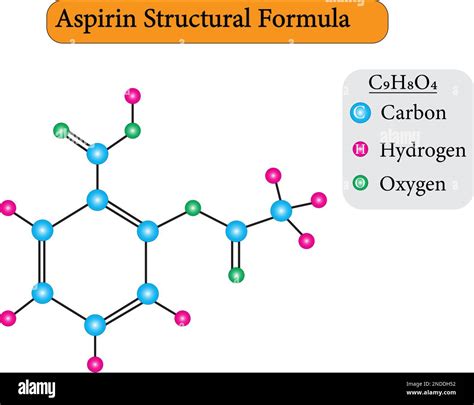
Lewis Structure of Aspirin
The Lewis structure of aspirin depicts the arrangement of its valence electrons, providing insights into its molecular geometry and bonding characteristics. The structure consists of a central carbon atom (C) bonded to two oxygen atoms (O), one with a single bond (C-O) and the other with a double bond (C=O); an acetyl group (-CH3=O) is attached to the remaining carbon atom, while a hydroxyl group (-OH) is bonded to the oxygen atom bearing the single bond.
O=C-O-C=O
| |
CH3 OH
Physical and Chemical Properties
Aspirin exhibits several physical and chemical properties that influence its behavior and applications:
- Molecular Weight: 180.15 g/mol
- Melting Point: 136-138 °C
- Boiling Point: 140 °C (decomposes)
- Solubility: Poorly soluble in cold water, moderately soluble in hot water, and soluble in organic solvents
- Acidity: Weak acid (pKa = 3.5)
- Antioxidant Activity: Aspirin possesses antioxidant properties, contributing to its potential health benefits
Applications of Aspirin
Aspirin’s diverse applications stem from its therapeutic properties and are widely employed in the following areas:
- Pain Relief: Aspirin is a well-known analgesic, effectively reducing pain caused by headaches, toothaches, and muscle strains.
- Inflammation Reduction: Its anti-inflammatory properties make it effective in treating various inflammatory conditions, including arthritis and bursitis.
- Fever Reduction: Aspirin acts as an antipyretic, reducing fever associated with infections and other medical conditions.
- Cardiovascular Health: Aspirin plays a role in preventing heart attacks and strokes by reducing inflammation and inhibiting platelet aggregation.
- Cancer Risk Reduction: Studies suggest that regular aspirin use may lower the risk of developing certain types of cancer, including colorectal and ovarian cancers.
Common Mistakes to Avoid When Using Aspirin
While aspirin is generally safe for most individuals, certain precautions are necessary:
- Overdosing: Exceeding the recommended dosage can lead to serious side effects, including stomach bleeding and liver damage.
- Stomach Irritation: Aspirin can irritate the stomach lining, especially in individuals with sensitive stomachs or ulcers.
- Interactions with Other Medications: Aspirin interacts with several medications, including anticoagulants, blood thinners, and other pain relievers; consulting a healthcare professional is crucial to avoid potential adverse effects.
- Pregnancy and Breastfeeding: Aspirin should be used cautiously during pregnancy and breastfeeding as it may pose risks to the fetus or infant.
Step-by-Step Approach for Understanding Aspirin
- Draw the Lewis Structure: As discussed earlier, accurately depicting the Lewis structure is essential to understand the molecular geometry and bonding characteristics of aspirin.
- Identify Functional Groups: Aspirin contains several functional groups, including a carboxylic acid (-COOH), an acetyl group (-CH3=O), and a hydroxyl group (-OH). Identifying these groups aids in analyzing the chemical properties of aspirin.
- Determine Molecular Properties: Calculate the molecular weight, melting point, boiling point, and solubility of aspirin based on its structure.
- Explore Applications: Research the diverse applications of aspirin in medicine, pharmaceuticals, and other industries.
- Consider Safety Precautions: Consult healthcare professionals and review relevant literature to understand potential side effects and interactions when using aspirin.
Future Applications
Aspirin’s versatility extends beyond its current applications, inspiring researchers to explore its potential in novel domains:
- Pain Management: Developing new aspirin formulations with enhanced pain-relieving properties and reduced side effects.
- Targeted Drug Delivery: Employing aspirin as a carrier molecule to deliver other therapeutic agents to specific body parts.
- Inflammation Research: Investigating aspirin’s molecular mechanisms to develop more effective anti-inflammatory treatments.
- Cardiovascular Health: Exploring the long-term effects of aspirin on cardiovascular health and identifying optimal dosage regimens for different patient populations.
- Preventive Medicine: Utilizing aspirin’s antioxidant and anti-cancer properties for preventive interventions against chronic diseases.
Tables
- Table 1: Physical Properties of Aspirin
| Property | Value |
|---|---|
| Molecular Weight | 180.15 g/mol |
| Melting Point | 136-138 °C |
| Boiling Point | 140 °C (decomposes) |
| Solubility | Poorly soluble in cold water, moderately soluble in hot water, soluble in organic solvents |
- Table 2: Chemical Properties of Aspirin
| Property | Value |
|---|---|
| Acidity | Weak acid (pKa = 3.5) |
| Anti-inflammatory Activity | Effective in reducing inflammation |
| Antioxidant Activity | Possesses antioxidant properties |
- Table 3: Applications of Aspirin
| Application | Description |
|---|---|
| Pain Relief | Reduces pain caused by headaches, toothaches, and muscle strains |
| Inflammation Reduction | Treats inflammatory conditions, including arthritis and bursitis |
| Fever Reduction | Lowers fever associated with infections and other medical conditions |
| Cardiovascular Health | Prevents heart attacks and strokes by reducing inflammation and inhibiting platelet aggregation |
| Cancer Risk Reduction | May lower the risk of developing certain types of cancer |
- Table 4: Common Mistakes to Avoid When Using Aspirin
| Mistake | Consequences |
|---|---|
| Overdosing | Stomach bleeding, liver damage |
| Stomach Irritation | Stomach lining irritation |
| Interactions with Other Medications | Potential adverse effects |
| Pregnancy and Breastfeeding | Risks to the fetus or infant |