Introduction
Ammonium is a ubiquitous chemical species present in various natural and industrial processes. Its acid-base properties, characterized by the dissociation constant known as Ka, play a vital role in numerous applications, ranging from fertilizer production to environmental monitoring. This article delves into the concept of Ka of NH4, exploring its significance, applications, and methods of measurement.

Definition of Ka
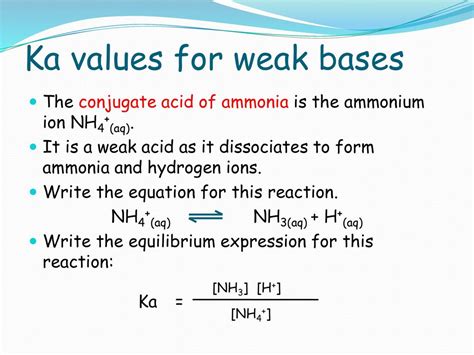
Ka is an equilibrium constant that quantifies the extent to which an acid dissociates in water. For weak acids, such as ammonium (NH4+), the dissociation process can be represented as:
NH4+ ⇌ H+ + NH3
The Ka value represents the equilibrium concentration of H+ ions relative to the concentration of undissociated NH4+ ions. A lower Ka value indicates a weaker acid, as it has a lower tendency to dissociate.
Significance of Ka in NH4+
The Ka of NH4+ is crucial in determining its acid-base behavior and subsequent applications. It governs the pH of aqueous solutions containing NH4+, affecting the solubility, reactivity, and biological processes that occur within them.
Applications of Ka in NH4+
The Ka of NH4+ finds applications in a diverse range of fields:
-
Fertilizer Production: Ammonium-based fertilizers, such as ammonium nitrate and ammonium sulfate, provide nitrogen to crops. The Ka value of NH4+ influences the solubility and uptake of nitrogen by plants.
-
Environmental Monitoring: NH4+ is a key indicator of nitrogen pollution in aquatic ecosystems. Monitoring the Ka of NH4+ in water samples helps assess the health of water bodies and implement appropriate remediation measures.
-
Pharmaceutical Industry: Ammonium salts are used as excipients in drug formulations. Understanding the Ka of NH4+ ensures the proper release and stability of drugs in the body.
-
Industrial Processes: NH4+ is involved in various industrial processes, such as the production of plastics, dyes, and explosives. The Ka value of NH4+ influences the reaction rates and product yields in these processes.
Methods of Measuring Ka
The Ka of NH4+ can be determined experimentally using several methods:
-
Potentiometry: This method measures the pH of a solution containing NH4+ and uses the pH and NH4+ concentration to calculate Ka.
-
Conductivity Measurements: The electrical conductivity of a solution containing NH4+ increases with increasing dissociation. By measuring the conductivity and knowing the NH4+ concentration, Ka can be determined.
-
Spectrophotometry: The absorbance of a solution containing NH4+ at a specific wavelength can be used to determine the concentration of undissociated NH4+. This information, along with the total NH4+ concentration, allows for the calculation of Ka.
Applications of Ka to Generate New Ideas
The concept of Ka can serve as a source of inspiration for generating new applications in various fields:
-
Agriculture: Developing fertilizers with customized Ka values to enhance nutrient uptake in specific soil conditions.
-
Water Treatment: Designing filtration systems that selectively remove NH4+ based on its Ka value, improving water quality.
-
Drug Delivery: Creating drug delivery systems that exploit the Ka of NH4+ to control drug release in different pH environments.
-
Industrial Processes: Optimizing reaction conditions in industrial processes involving NH4+ by manipulating its Ka value to improve yields and efficiency.
Data Tables
Table 1: Ka Values of Weak Acids
| Acid | Ka |
|---|---|
| Acetic Acid (CH3COOH) | 1.8 x 10^-5 |
| Ammonium (NH4+) | 5.6 x 10^-10 |
| Carbonic Acid (H2CO3) | 4.5 x 10^-7 |
| Formic Acid (HCOOH) | 1.8 x 10^-4 |
| Hydrofluoric Acid (HF) | 6.8 x 10^-4 |
Table 2: Applications of Ka in NH4+
| Application | Description |
|---|---|
| Fertilizer Production | Optimizing nitrogen uptake by crops |
| Environmental Monitoring | Assessing water quality and nitrogen pollution |
| Pharmaceutical Industry | Ensuring proper drug release and stability |
| Industrial Processes | Influencing reaction rates and product yields |
Table 3: Methods of Measuring Ka
| Method | Principle |
|---|---|
| Potentiometry | Measuring pH and NH4+ concentration |
| Conductivity Measurements | Measuring electrical conductivity |
| Spectrophotometry | Determining undissociated NH4+ concentration |
Table 4: New Ideas Generated from Ka
| Field | Idea |
|---|---|
| Agriculture | Customized fertilizers with tailored Ka values |
| Water Treatment | Selective NH4+ removal based on Ka |
| Drug Delivery | pH-responsive drug delivery systems using Ka |
| Industrial Processes | Optimizing reaction conditions using Ka |
Conclusion
The Ka of NH4+ plays a pivotal role in a myriad of applications. Understanding its significance, methods of measurement, and potential for generating new ideas empowers scientists and engineers to harness the acid-base properties of ammonium for solving real-world problems. By exploring the Ka of NH4+, we unlock the potential for advancements in agriculture, environmental monitoring, pharmaceuticals, and industrial processes.
Frequently Asked Questions (FAQs)
-
What is the difference between Ka and pKa?
Ka is the equilibrium constant in terms of molarity, while pKa is its negative logarithm (pKa = -log(Ka)). -
Why is Ka important in fertilizer production?
Ka determines the availability of nitrogen to plants by influencing the solubility and uptake of ammonium-based fertilizers. -
How can Ka be used in environmental monitoring?
Ka helps determine the extent of nitrogen pollution in water bodies, guiding remediation efforts. -
What is the role of Ka in the pharmaceutical industry?
Ka influences drug release and stability, ensuring optimal therapeutic effects. -
How can Ka be utilized in industrial processes?
Ka can be manipulated to optimize reaction conditions, improving yields and efficiency. -
What are some ideas for new applications of Ka in NH4+?
– Customized fertilizers for specific soil conditions
– Selective removal of NH4+ from wastewater
– pH-responsive drug delivery systems
– Optimizing reaction conditions in industrial processes -
How can the Ka of NH4+ be measured?
Ka can be determined using potentiometry, conductivity measurements, or spectrophotometry. -
How does Ka contribute to scientific advancement?
Understanding Ka enables researchers and engineers to develop innovative applications in agriculture, environmental monitoring, pharmaceuticals, and industrial processes.