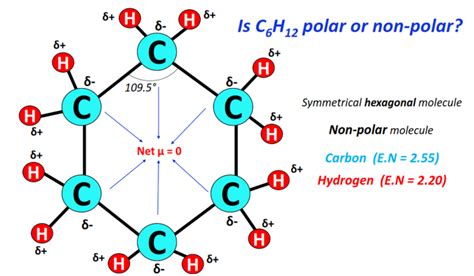
Cyclohexane is a cyclic hydrocarbon with the molecular formula C6H12. It is a colorless liquid with a characteristic gasoline-like odor. Cyclohexane is a nonpolar molecule, meaning that it does not have a net electrical charge. This is because the carbon atoms in the ring are bonded to each other by covalent bonds, which are nonpolar. The hydrogen atoms are also bonded to the carbon atoms by covalent bonds, which are also nonpolar.

The nonpolarity of cyclohexane is important for its physical and chemical properties. For example, cyclohexane is immiscible with water, meaning that it does not dissolve in water. This is because water is a polar molecule, and cyclohexane is a nonpolar molecule. Polar molecules and nonpolar molecules do not mix well together.
Cyclohexane is also a good solvent for nonpolar compounds. This is because nonpolar compounds dissolve in nonpolar solvents. Cyclohexane is often used as a solvent for paints, oils, and greases.
Applications of Cyclohexane
Cyclohexane is used in a variety of applications, including:
- As a solvent: Cyclohexane is a good solvent for nonpolar compounds. It is often used as a solvent for paints, oils, and greases.
- As a fuel: Cyclohexane can be used as a fuel for internal combustion engines. It is a high-octane fuel, meaning that it burns cleanly and efficiently.
- As a raw material: Cyclohexane is used as a raw material for the production of other chemicals, such as nylon and caprolactam.
Physical and Chemical Properties of Cyclohexane
The physical and chemical properties of cyclohexane are as follows:
| Property | Value |
|---|---|
| Molecular formula | C6H12 |
| Molecular weight | 84.16 g/mol |
| Density | 0.779 g/cm³ |
| Melting point | 6.5 °C |
| Boiling point | 80.7 °C |
| Flash point | -18 °C |
| Autoignition temperature | 260 °C |
| Explosive limits | 1.3–8.0% |
Safety Considerations
Cyclohexane is a flammable liquid. It is important to take precautions to prevent fires when working with cyclohexane. Cyclohexane is also a skin irritant. It is important to wear gloves and protective clothing when working with cyclohexane.
Tips and Tricks
Here are some tips and tricks for working with cyclohexane:
- Always keep cyclohexane away from heat and open flames.
- Always wear gloves and protective clothing when working with cyclohexane.
- If cyclohexane comes into contact with your skin, wash it off immediately with soap and water.
- If cyclohexane is ingested, do not induce vomiting. Call a doctor immediately.
Common Mistakes to Avoid
Here are some common mistakes to avoid when working with cyclohexane:
- Do not heat cyclohexane in a closed container. This could cause the container to explode.
- Do not use cyclohexane as a solvent for polar compounds. Polar compounds will not dissolve in cyclohexane.
- Do not store cyclohexane in a plastic container. Cyclohexane can dissolve plastic.
FAQs
Here are some frequently asked questions about cyclohexane:
- Is cyclohexane polar? No, cyclohexane is not polar.
- What is the molecular formula of cyclohexane? The molecular formula of cyclohexane is C6H12.
- What is the boiling point of cyclohexane? The boiling point of cyclohexane is 80.7 °C.
- Is cyclohexane flammable? Yes, cyclohexane is flammable.
- What are the safety precautions for working with cyclohexane? Always keep cyclohexane away from heat and open flames. Always wear gloves and protective clothing when working with cyclohexane.