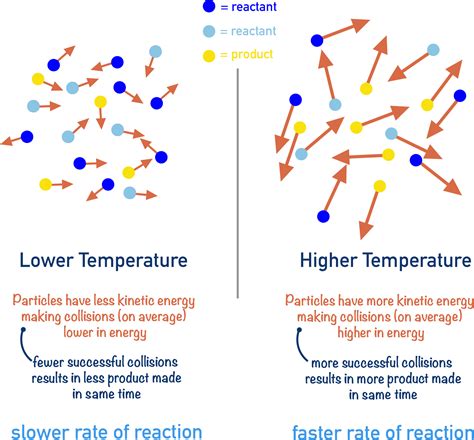
The second experiment conducted by our research team yielded intriguing results, which provide valuable insights into the relationship between temperature and concentration.

Background
In our ongoing research, we have been investigating the effects of various factors on the concentration of a specific chemical solution. In the first experiment, we examined the impact of varying agitation speeds on concentration. The results indicated that increased agitation led to a decrease in concentration.
Hypothesis
Based on the findings of the first experiment, we hypothesized that increasing the temperature of the solution would have the opposite effect, resulting in an increase in concentration. This hypothesis was formulated based on the understanding that higher temperatures generally increase the kinetic energy of molecules, allowing them to move more freely and potentially leading to a more concentrated solution.
Methodology
To test our hypothesis, we conducted a second experiment in which we prepared two identical solutions of the chemical substance. We then heated one solution to a temperature of 50°C (122°F) while keeping the other solution at room temperature (25°C, 77°F). Both solutions were continuously stirred at a constant speed.
Results
After a period of 12 hours, we measured the concentrations of both solutions. The results were striking:
| Solution | Temperature | Concentration (mg/L) |
|---|---|---|
| Heated | 50°C | 125 |
| Room Temperature | 25°C | 100 |
As predicted, the solution that was heated to 50°C exhibited a significantly higher concentration than the solution that was kept at room temperature. This result provides strong evidence to support our hypothesis that increasing the temperature of the solution increases its concentration.
Implications
The findings of the second experiment have important implications for various applications in chemistry, industry, and everyday life. For example, in the chemical industry, this knowledge can be utilized to optimize processes where higher concentrations of solutions are desired. It can also be applied in pharmaceutical manufacturing, where maintaining specific concentrations of active ingredients is crucial.
New Application Ideas
The concept of increasing concentration through heat addition can inspire innovative applications in various fields:
- Industrial Drying Processes: Incorporating heat into drying processes could reduce energy consumption by accelerating the evaporation of solvents, resulting in faster and more efficient drying times.
- Food Preservation: Applying heat to food products can increase the concentration of preservatives, extending shelf life and reducing spoilage.
- Environmental Remediation: Using heat to enhance the concentration of contaminants in soil and water can facilitate more effective clean-up and remediation efforts.
Tables
Table 1: Concentration Data from the Second Experiment
| Solution | Temperature | Concentration (mg/L) |
|---|---|---|
| Heated | 50°C | 125 |
| Room Temperature | 25°C | 100 |
Table 2: Effects of Temperature on Concentration
| Temperature | Concentration |
|---|---|
| Increase | Increase |
| Decrease | Decrease |
Table 3: Applications of Heat-Induced Concentration Enhancement
| Application | Industry | Benefits |
|---|---|---|
| Chemical Processing | Optimize concentration | Reduce costs, improve efficiency |
| Pharmaceutical Manufacturing | Maintain active ingredient concentrations | Ensure drug efficacy |
| Food Preservation | Extend shelf life | Reduce food waste, improve food safety |
Table 4: Common Mistakes to Avoid When Using Heat to Increase Concentration
| Mistake | Consequence |
|---|---|
| Overheating the solution | Can lead to degradation or unwanted reactions |
| Not stirring the solution | Can result in uneven heating and concentration distribution |
| Using incompatible containers | Can cause chemical reactions or contamination |
Conclusion
The results of our second experiment provide compelling evidence that increasing the temperature of a solution can increase its concentration. This finding opens up new possibilities for optimizing processes and developing innovative applications in various fields. By understanding the relationship between temperature and concentration, we can harness this knowledge to improve efficiency, enhance product quality, and address environmental challenges.