Chemical reactions involve the making and breaking of chemical bonds. These processes require energy input or release. Understanding whether a reaction is endothermic or exothermic is crucial for industrial processes, drug development, and everyday life. Here’s a comprehensive guide to help you differentiate between endothermic and exothermic reactions.

What is an Endothermic Reaction?
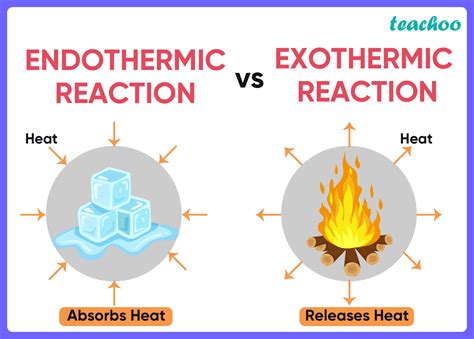
An endothermic reaction absorbs heat from its surroundings to proceed.
The products of the reaction have higher potential energy than the reactants. As a result, the temperature of the surroundings decreases during an endothermic reaction.
Example:
– Melting of ice
– Dissolving ammonium chloride in water
What is an Exothermic Reaction?
An exothermic reaction releases heat into its surroundings.
The products of the reaction have lower potential energy than the reactants. As a result, the temperature of the surroundings increases during an exothermic reaction.
Example:
– Burning of fuel
– Precipitation of calcium carbonate
How to Determine if a Reaction is Endothermic or Exothermic
Method 1: Calorimetry
Calorimetry is the most direct method to determine the nature of a reaction. A calorimeter measures the heat flow between the reaction and its surroundings.
Steps:
1. Place the reactants in a calorimeter.
2. Initiate the reaction.
3. Record the temperature change (ΔT) of the surroundings.
- If ΔT > 0, the reaction is exothermic.
- If ΔT < 0, the reaction is endothermic.
Method 2: Enthalpy Change (ΔH)
The enthalpy change (ΔH) is the amount of heat absorbed or released during a reaction. According to thermodynamics, for a reaction:
ΔH > 0: Endothermic reaction
ΔH < 0: Exothermic reaction
Common Mistakes to Avoid
Mistake 1: Equating Heat Release with Exothermic
Not all heat-releasing reactions are exothermic. For example, when water condenses, heat is released, but the reaction is exothermic because the water molecules lose potential energy as they form bonds.
Mistake 2: Assuming All Reactions are Either Endothermic or Exothermic
Some reactions can be both endothermic and exothermic, depending on the conditions. For example, the Haber process for ammonia production is exothermic at low temperatures but endothermic at high temperatures.
Step-by-Step Approach to Identify Endothermic/Exothermic Reactions
Step 1: Identify the Reactants and Products
Step 2: Calculate the Enthalpy Change (ΔH)
– If ΔH > 0, the reaction is endothermic.
– If ΔH < 0, the reaction is exothermic.
Additional Considerations
Effect of Temperature
Temperature can affect the endothermic/exothermic nature of a reaction. An endothermic reaction at low temperatures may become exothermic at higher temperatures.
Reversible Reactions
Reversible reactions can proceed in both directions, releasing heat (exothermic) in one direction and absorbing heat (endothermic) in the opposite direction.
Applications of Endothermic and Exothermic Reactions
Endothermic Reactions:
- Air conditioning and refrigeration
- Dissolving heat packs
- Cooling chemical processes
Exothermic Reactions:
- Rocket propellants
- Explosives
- Firewood combustion
FAQs
Q1: How can you measure the heat flow in a reaction?
A1: Calorimetry, using a device called a calorimeter.
Q2: What is the difference between endothermic and exothermic enthalpy changes?
A2: Endothermic reactions have ΔH > 0, while exothermic reactions have ΔH < 0.
Q3: Can a reaction be both endothermic and exothermic?
A3: Yes, depending on the conditions (e.g., temperature).
Q4: What are some common endothermic reactions?
A4: Melting ice, dissolving ammonium chloride in water.
Q5: What are some common exothermic reactions?
A5: Burning fuel, precipitation of calcium carbonate.
Q6: How can the enthalpy change of a reaction be predicted?
A6: Using Hess’s law or the bond enthalpy method.
Conclusion
Determining the endothermic or exothermic nature of a reaction is crucial for understanding its behavior and applications. By using calorimetry or calculating the enthalpy change, chemists can differentiate between these two types of reactions. This knowledge is essential in various fields, including industrial chemistry, drug development, and everyday life.