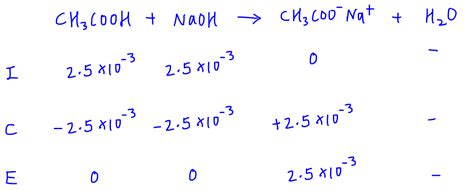The equivalence point in a titration is where the moles of acid and base are equal. At this point, the solution is neutralized and the pH is 7.00.

To calculate the pH at the equivalence point, you need to know the concentration of the acid and base and the volume of each solution.
Step 1: Calculate the moles of acid and base
To calculate the moles of acid and base, you need to multiply the concentration of each solution by its volume.
moles = concentration × volume
Step 2: Set up an ICE table
An ICE table is a tool that helps you keep track of the changes in concentration of the reactants and products in a chemical reaction.
The ICE table for the reaction between a strong acid and a strong base is as follows:
| Reactant | Initial | Change | Equilibrium |
|---|---|---|---|
| Acid | A | -A | A-A |
| Base | B | -B | B-B |
| H3O+ | 0 | +A | A |
| OH- | 0 | +B | B |
Step 3: Solve for A or B
Since the moles of acid and base are equal at the equivalence point, you can set A = B.
A = B
Step 4: Calculate the concentration of H3O+
The concentration of H3O+ is equal to the concentration of OH- at the equivalence point.
[H3O+] = [OH-]
Step 5: Calculate the pH
The pH is equal to the negative log of the concentration of H3O+.
pH = -log[H3O+]
Example
Let’s say you have a titration of 50.0 mL of 0.100 M HCl with 50.0 mL of 0.100 M NaOH.
Step 1: Calculate the moles of acid and base
moles of HCl = 0.100 M × 50.0 mL = 0.00500 mol moles of NaOH = 0.100 M × 50.0 mL = 0.00500 mol
Step 2: Set up an ICE table
| Reactant | Initial | Change | Equilibrium |
|---|---|---|---|
| HCl | 0.00500 mol | -0.00500 mol | 0 mol |
| NaOH | 0.00500 mol | -0.00500 mol | 0 mol |
| H3O+ | 0 | +0.00500 mol | 0.00500 mol |
| OH- | 0 | +0.00500 mol | 0.00500 mol |
Step 3: Solve for A or B
A = B = 0.00500 mol
Step 4: Calculate the concentration of H3O+
[H3O+] = 0.00500 mol / 100.0 mL = 0.000100 M
Step 5: Calculate the pH
pH = -log(0.000100 M) = 4.00
Tips and Tricks
- It is important to make sure that the concentrations of the acid and base are equal at the equivalence point. If they are not, the pH will not be 7.00.
- You can use a pH meter to measure the pH of the solution at the equivalence point.
- If you are titrating a weak acid or base, you will need to use a different formula to calculate the pH at the equivalence point.
Tables
| Acid | Base | Equivalence Point pH |
|---|---|---|
| Strong acid | Strong base | 7.00 |
| Weak acid | Strong base | >7.00 |
| Strong acid | Weak base | <7.00 |
| Weak acid | Weak base | Depends on the dissociation constants of the acid and base |
Applications
The pH at the equivalence point is important in a variety of applications, including:
- Titrations
- Acid-base reactions
- Buffer solutions
- Water treatment
Conclusion
The pH at the equivalence point is a useful tool for understanding and controlling chemical reactions. By following the steps outlined in this article, you can easily calculate the pH at the equivalence point for any acid-base titration.