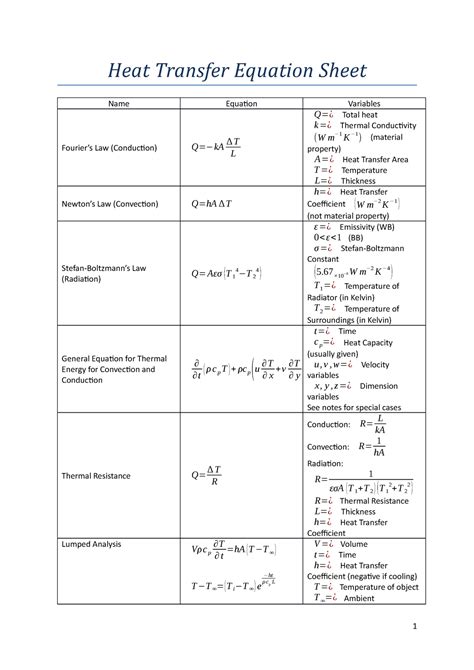
Heat transfer is the movement of thermal energy from one object or substance to another. It is a fundamental process in many engineering and scientific applications, such as the design of heating and cooling systems, power plants, and chemical reactors.

There are three main modes of heat transfer: conduction, convection, and radiation.
Conduction is the transfer of heat through direct contact between two objects or substances. The rate of heat transfer by conduction is proportional to the temperature difference between the two objects, the area of contact, and the thermal conductivity of the material.
Convection is the transfer of heat through the movement of a fluid (liquid or gas). The rate of heat transfer by convection is proportional to the temperature difference between the fluid and the surface, the area of the surface, the velocity of the fluid, and the specific heat capacity of the fluid.
Radiation is the transfer of heat through electromagnetic waves. The rate of heat transfer by radiation is proportional to the temperature difference between the two objects, the area of the surfaces, and the emissivity of the surfaces.
The following table summarizes the three modes of heat transfer and their governing equations:
| Mode of Heat Transfer | Governing Equation |
|---|---|
| Conduction | $Q = kA(T_h – T_c)$ |
| Convection | $Q = hA(T_s – T_f)$ |
| Radiation | $Q = \epsilon \sigma A(T_h^4 – T_c^4)$ |
where:
- $Q$ is the rate of heat transfer (W)
- $k$ is the thermal conductivity (W/m-K)
- $A$ is the area of the surface (m2)
- $T_h$ is the temperature of the hotter object (K)
- $T_c$ is the temperature of the colder object (K)
- $h$ is the convective heat transfer coefficient (W/m2-K)
- $T_s$ is the temperature of the surface (K)
- $T_f$ is the temperature of the fluid (K)
- $\epsilon$ is the emissivity of the surface
- $\sigma$ is the Stefan-Boltzmann constant (5.67 x 10-8 W/m2-K4)
Applications of Heat Transfer
Heat transfer is a fundamental process in many engineering and scientific applications. Some of the most common applications include:
- Heating and cooling systems: Heat transfer is used to control the temperature of buildings, vehicles, and other enclosed spaces.
- Power plants: Heat transfer is used to generate electricity from fossil fuels, nuclear energy, and renewable energy sources.
- Chemical reactors: Heat transfer is used to control the temperature of chemical reactions and to prevent them from overheating.
- Electronics: Heat transfer is used to cool electronic components and to prevent them from overheating.
- Medical devices: Heat transfer is used to heat and cool medical devices, such as MRI machines and x-ray machines.
New Applications of Heat Transfer
The field of heat transfer is constantly evolving, and new applications are being developed all the time. Some of the most promising new applications of heat transfer include:
- Thermal energy storage: Heat transfer is used to store thermal energy in materials, such as molten salt or concrete. This energy can then be used to generate electricity or to heat buildings and vehicles.
- Nanotechnology: Heat transfer is used to create and manipulate nanomaterials, which have unique thermal properties. These materials can be used to create new types of heat exchangers, thermal sensors, and other devices.
- Biomimetics: Heat transfer is used to study the thermal properties of living organisms. This knowledge can be used to develop new biomimetic materials and devices, such as artificial skin and self-healing materials.
Tables
The following tables provide additional information on heat transfer.
Table 1: Thermal Conductivity of Common Materials
| Material | Thermal Conductivity (W/m-K) |
|---|---|
| Aluminum | 237 |
| Copper | 401 |
| Gold | 318 |
| Iron | 80 |
| Steel | 50 |
| Water | 0.6 |
| Air | 0.024 |
Table 2: Convective Heat Transfer Coefficients for Common Fluids
| Fluid | Convective Heat Transfer Coefficient (W/m2-K) |
|---|---|
| Air | 10-100 |
| Water | 100-1000 |
| Oil | 10-100 |
| Glycol | 100-1000 |
Table 3: Emissivity of Common Materials
| Material | Emissivity |
|---|---|
| Aluminum | 0.05-0.15 |
| Copper | 0.05-0.10 |
| Gold | 0.02-0.05 |
| Iron | 0.20-0.30 |
| Steel | 0.20-0.30 |
| Water | 0.95-1.00 |
| Air | 0.00-0.10 |
Table 4: Specific Heat Capacity of Common Materials
| Material | Specific Heat Capacity (J/kg-K) |
|---|---|
| Aluminum | 900 |
| Copper | 385 |
| Gold | 129 |
| Iron | 450 |
| Steel | 460 |
| Water | 4187 |
| Air | 1005 |