Introduction
The HCONH2 Lewis structure, also known as formamide, is a crucial molecule in various chemical and biological processes. Understanding its structure is essential for comprehending its properties, reactivity, and applications. This article delves into the HCONH2 Lewis structure, explaining its key features, hybridization, and molecular geometry.
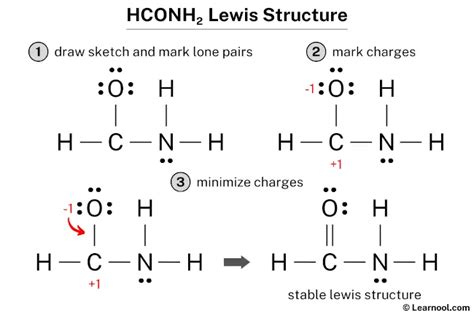
HCONH2 Lewis Structure: A Step-by-Step Guide
To draw the HCONH2 Lewis structure, follow these steps:
- Count the total number of valence electrons: H (1) + C (4) + O (6) + N (5) + H (1) = 17
- Place the least electronegative atom (carbon) in the center and arrange the other atoms around it.
- Connect the atoms with single bonds (two shared electrons per bond) to satisfy the octet rule for carbon and oxygen.
- Place the remaining hydrogen atoms to satisfy the octet rule for nitrogen.
- Check the formal charges to ensure they are all zero.
The resulting Lewis structure is:
H-C=O
|
N-H
Hybridization and Molecular Geometry
The carbon atom in HCONH2 undergoes sp2 hybridization, meaning it forms three sigma bonds in a trigonal planar geometry. The oxygen atom forms two sigma bonds and two lone pairs, resulting in a bent geometry. The nitrogen atom also undergoes sp2 hybridization, forming three sigma bonds and one lone pair, leading to a trigonal pyramidal geometry.
Resonance Structures
HCONH2 can exhibit resonance, which occurs when multiple valid Lewis structures can be drawn for a molecule. The resonance structures of HCONH2 are:
H-C=O H-C-O
| ||
N-H N-H
These resonance structures contribute to the overall stability and reactivity of the molecule.
Properties and Applications
Properties:
- Boiling point: 210.5 °C
- Melting point: -47 °C
- Density: 1.133 g/mL
- Polarity: Polar
Applications:
- Solvent in chemical reactions
- Precursor to other chemicals, such as urea
- Building block in pharmaceutical drugs
- Component in cosmetics and personal care products
Related Compounds
Formamide Derivatives:
- N-Methylformamide
- N,N-Dimethylformamide
- N-Formylpiperidine
Other Amides:
- Acetamide
- Propionamide
- Butyramide
Tips and Tricks for Understanding HCONH2
- Remember that the least electronegative atom is typically placed in the center of the Lewis structure.
- Use the octet rule to ensure that all atoms have a stable electron configuration.
- Consider resonance to account for multiple possible Lewis structures.
- Understand the hybridization of the atoms to determine their molecular geometry.
- Explore the properties and applications of HCONH2 to appreciate its practical significance.
Conclusion
The HCONH2 Lewis structure provides a fundamental understanding of the molecular interactions and properties of formamide. By studying its structure, hybridization, and resonance, researchers and students can gain insights into the behavior and applications of this versatile molecule.
Additional Information
Table 1: Properties of HCONH2
| Property | Value |
|---|---|
| Molecular weight | 45.04 g/mol |
| Solubility in water | Miscible |
| Dipole moment | 3.72 D |
Table 2: Applications of HCONH2
| Application | Industry |
|---|---|
| Solvent | Pharmaceutical |
| Precursor to urea | Chemical |
| Building block in drugs | Pharmaceutical |
| Component in cosmetics | Personal care |
Table 3: Resonance Structures of HCONH2
| Resonance Structure | Formal Charges |
|---|---|
| H-C=O | N: -1 |
| N-H | |
| H-C-O | |
Table 4: Related Amides
| Amide | Molecular Formula |
|---|---|
| Acetamide | CH3CONH2 |
| Propionamide | C2H5CONH2 |
| Butyramide | C3H7CONH2 |