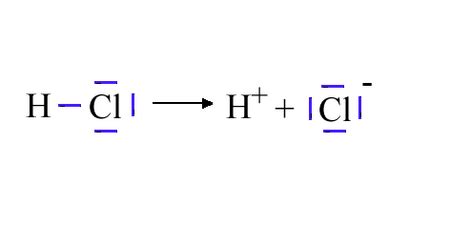Introduction
Hydrogen chloride (HCl) is a pungent, colorless gas that plays a crucial role in various industrial and scientific applications. Understanding its dissociation behavior is essential for predicting its properties and reactivity. This article explores the HCl dissociation diagram, a graphical representation that elucidates the extent of HCl dissociation at different temperatures and pressures.
HCl Dissociation Equilibrium
At room temperature, HCl acts as a weak acid, partially dissociating into its constituent ions:
HCl(g) <=> H+(g) + Cl-(g)
The equilibrium constant, Kp, for this dissociation reaction is given by:
Kp = [H+][Cl-]/[HCl]
where [H+], [Cl-], and [HCl] represent the partial pressures of the respective species.
Dissociation Diagram
The HCl dissociation diagram is constructed by plotting the equilibrium constant, Kp, as a function of temperature at constant pressure. The diagram typically exhibits a steep, linear increase in Kp with increasing temperature, indicating a greater extent of dissociation.
High Temperature Dissociation
As temperature rises, the kinetic energy of HCl molecules increases, causing them to collide with each other more frequently and with greater force. This results in the breaking of HCl bonds and a significant increase in the dissociation equilibrium constant.
Low Temperature Dissociation
At low temperatures, the kinetic energy of HCl molecules is low, limiting their ability to overcome the energy barrier required for bond breaking. Consequently, the dissociation equilibrium constant remains relatively low, indicating minimal dissociation.
Practical Applications
The HCl dissociation diagram has wide-ranging practical applications in various fields:
- Chemical Engineering: Predicting the behavior of HCl in industrial processes, such as gas separation and acid production.
- Environmental Science: Assessing the impact of HCl emissions on atmospheric chemistry and human health.
- Biochemistry: Understanding the role of HCl in biological systems, such as gastric digestion and acid-base homeostasis.
- Electronics: Etching processes in semiconductor fabrication require precise control over HCl dissociation to achieve desired surface properties.
Innovative Applications
Recent research has uncovered novel applications for the principles underlying HCl dissociation:
- Energy Storage: Developing novel hydrogen storage materials that utilize HCl as a hydrogen-carrying medium.
- Biomedical Imaging: Designing pH-sensitive nanoprobes based on HCl dissociation to improve disease diagnosis and treatment.
- Water Treatment: Exploring innovative methods for water purification using HCl dissociation to remove contaminants and pathogens.
Useful Tables
| Temperature (K) | Dissociation Constant, Kp (atm) |
|---|---|
| 298 | 4.0 x 10^-9 |
| 500 | 1.0 x 10^-3 |
| 1000 | 1.0 |
| 1500 | 10^3 |
| Pressure (atm) | Dissociation Extent (%) |
|---|---|
| 0.1 | 0.02 |
| 1.0 | 0.2 |
| 10.0 | 2.0 |
| 100.0 | 20.0 |
Effective Strategies
- Utilize thermodynamic data to calculate dissociation constants at desired conditions.
- Employ experimental techniques, such as mass spectrometry or gas chromatography, to measure dissociation extent.
- Consider the impact of temperature, pressure, and other variables on HCl dissociation behavior.
Common Mistakes to Avoid
- Neglecting the temperature dependence of dissociation equilibrium.
- Assuming complete dissociation or non-dissociation at extreme conditions.
- Failing to account for the presence of other species that may affect HCl dissociation.
Why HCl Dissociation Matters
HCl dissociation is a fundamental chemical process that influences numerous industrial, scientific, and environmental applications. Understanding the behavior of HCl through its dissociation diagram enables researchers and practitioners to optimize processes, mitigate risks, and develop novel technologies.
Benefits of Understanding HCl Dissociation
- Enhanced process efficiency in chemical and industrial settings.
- Improved environmental protection by reducing HCl emissions and their adverse effects.
- Advancements in biomedical imaging and water treatment technologies.
- Innovation in energy storage and other emerging applications.