Introduction

Fluorine and bromine, two highly reactive elements, form a unique and volatile chemical compound called bromine pentafluoride (BrF5). This interhalogen compound exhibits remarkable properties and finds diverse applications across various industries. Delving into its chemical formula, properties, and applications will unveil the intriguing nature of this compound.
Chemical Formula and Structure
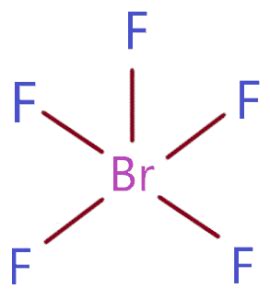
The chemical formula of bromine pentafluoride is BrF5. It consists of one central bromine atom (Br) bonded to five fluorine atoms (F). The molecular structure adopts a trigonal bipyramidal geometry, with the bromine atom occupying the axial position and the fluorine atoms occupying the equatorial positions.
Physical and Chemical Properties
Physical Properties:
- Appearance: Colorless to pale yellow gas
- Melting point: -79.8 °C
- Boiling point: 126.5 °C
- Density: 3.8 g/L (at 25 °C)
Chemical Properties:
- Highly reactive and corrosive
- Oxidizing agent
- Forms adducts with Lewis acids and bases
- Reacts with water to form hydrofluoric acid (HF) and bromic acid (HBrO3)
Synthesis and Production
Bromine pentafluoride is typically synthesized through the reaction of bromine (Br2) with fluorine gas (F2) under controlled conditions. This exothermic reaction yields BrF5 as a byproduct along with other interhalogen compounds.
Applications and Uses
Industrial Processes:
- Oxidizing agent in chemical synthesis and material purification
- Etching agent for semiconductors and electronics
- Catalyst for polymerization reactions
- Fluorination agent in uranium enrichment
Laboratory Applications:
- Preparative reagent for organofluorine compounds
- Solvent for ionic liquids
- Analytical reagent for trace analysis
Emerging Applications:
- Energy storage: Promising candidate for electrochemical cells due to its high oxidizing potential
- Medical imaging: Potential contrast agent in computed tomography (CT) scans
- Aerospace: Oxidizer in rocket propellants
- Water treatment: Oxidant for removing contaminants and pathogens
Safety Considerations and Handling
Due to its highly reactive and corrosive nature, bromine pentafluoride requires rigorous safety measures during handling and storage.
- Use in well-ventilated areas with appropriate personal protective equipment (PPE)
- Handle in specialized, leak-proof containers
- Avoid contact with moisture and organic materials
- Store in a cool, dry, and well-ventilated area away from incompatible substances
Tables
| Property | Value |
|---|---|
| Molecular Formula | BrF5 |
| Molecular Weight | 174.91 g/mol |
| Melting Point | -79.8 °C |
| Boiling Point | 126.5 °C |
| Density (25 °C) | 3.8 g/L |
| Application | Industry |
|---|---|
| Oxidizing Agent | Chemical Synthesis, Material Purification |
| Etching Agent | Semiconductor, Electronics |
| Catalyst | Polymerization Reactions |
| Fluorination Agent | Uranium Enrichment |
| Safety Measure | Description |
|---|---|
| Ventilation | Use in well-ventilated areas |
| Personal Protective Equipment | Gloves, Safety Glasses, Respirator |
| Container Requirements | Leak-proof, Specialized |
| Storage Conditions | Cool, Dry, Ventilated |
| Incompatible Substances | Moisture, Organic Materials |
| Application (Emerging) | Potential Industry |
|---|---|
| Energy Storage | Electrochemical Cells |
| Medical Imaging | Computed Tomography (CT) |
| Aerospace | Rocket Propellants |
| Water Treatment | Contaminant Removal |