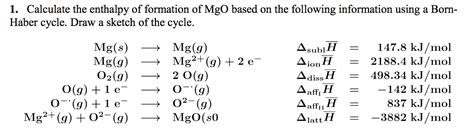Introduction
Magnesium oxide (MgO), commonly known as magnesia, is an inorganic compound with intriguing properties and diverse industrial applications. One of its fundamental characteristics is its enthalpy of formation, which plays a crucial role in understanding its reactivity and energy requirements. This article delves into the enthalpy of formation of MgO, exploring its significance, measurement techniques, and potential applications.

Enthalpy of Formation: A Fundamental Concept
Enthalpy of formation is a thermodynamic property that measures the energy change associated with the formation of one mole of a compound from its constituent elements in their standard states. For MgO, the enthalpy of formation represents the energy required to convert one mole of magnesium and one mole of oxygen into one mole of MgO.
Measurement Techniques
The enthalpy of formation of MgO can be determined using various experimental techniques, including:
- Calorimetry: This method involves measuring the heat released or absorbed during the formation of MgO.
- Electrochemical Methods: By measuring the electromotive force of cells involving the formation of MgO, the enthalpy change can be calculated.
- Computational Methods: Using computer simulations, the enthalpy of formation can be predicted based on the electronic structure and bonding interactions of the compound.
Value and Significance
The enthalpy of formation of MgO is -601.7 kJ/mol, indicating that the formation of MgO from its elements is an exothermic process. This negative value reflects the stability of MgO and its strong bonding between the magnesium and oxygen atoms. The enthalpy of formation is not only a fundamental property but also has practical implications:
- Estimation of Reaction Energies: By knowing the enthalpies of formation of the reactants and products, the enthalpy change for a chemical reaction can be predicted.
- Thermochemical Calculations: It enables the calculation of other thermodynamic properties, such as entropy and Gibbs free energy, which are essential for process optimization and material design.
- Industrial Applications: The enthalpy of formation is used to evaluate the energy efficiency of processes involving MgO, such as in refractory materials, cement production, and waste treatment.
Potential Applications
The unique properties and low enthalpy of formation make MgO a promising material for a wide range of applications:
- Refractories: MgO’s high thermal stability and resistance to corrosion make it an ideal material for lining furnaces and kilns in industries such as steel and glass production.
- Cement Production: MgO acts as a flux in the production of cement, reducing the clinkering temperature and improving the strength and durability of the final product.
- Waste Treatment: MgO can be used as a neutralizing agent for acidic waste streams, such as in the treatment of wastewater from industrial processes.
- Biomedical Materials: The biocompatibility and osteoconductivity of MgO make it a potential candidate for bone implants and tissue engineering applications.
- Hydrogen Storage: MgO can be used as a hydrogen storage medium due to its ability to form magnesium hydride (MgH2) under high pressure.
Tables
| Table 1: Enthalpy of Formation of MgO |
|---|
| Source |
| National Institute of Standards and Technology (NIST) |
| ThermoCalc Software |
| Barin, I. & Knacke, O. (1973) Thermochemical Properties of Inorganic Substances |
| Table 2: Industrial Applications of MgO |
| —————————————————————————– |
| Application |
| Refractories |
| Cement Production |
| Waste Treatment |
| Biomedical Materials |
| Hydrogen Storage |
| Table 3: Measurement Techniques for Enthalpy of Formation of MgO |
| —————————————————————————– |
| Technique |
| Calorimetry |
| Electrochemical Methods |
| Computational Methods |
| Table 4: Factors Influencing Enthalpy of Formation of MgO |
| —————————————————————————– |
| Factor |
| Bonding Strength |
| Crystal Structure |
| Particle Size |
| Temperature |
| Impurities |
Conclusion
The enthalpy of formation of MgO is a fundamental thermodynamic property that provides valuable insights into its stability, reactivity, and potential applications. Its exothermic nature and low value make MgO an attractive material for various industries. By understanding and harnessing the enthalpy of formation, researchers and engineers can optimize processes, develop innovative materials, and address critical challenges across diverse fields.
Additional Resources
- NIST Chemistry WebBook
- ThermoCalc Software
- Barin, I. & Knacke, O. (1973) Thermochemical Properties of Inorganic Substances