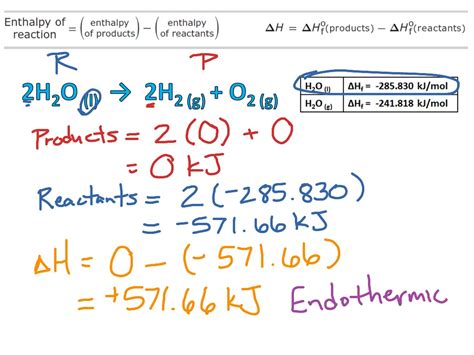
Understanding Enthalpy Change
When a chemical reaction occurs, energy is either released or absorbed. This energy change is known as enthalpy change, often abbreviated as ΔH. Enthalpy is a thermodynamic property that measures the total thermal energy of a system, including its internal energy and the work done by the system on its surroundings.

Enthalpy Change Units
Enthalpy change is typically measured in kilojoules per mole (kJ/mol). This unit represents the amount of energy released or absorbed per mole of the limiting reactant in a chemical reaction. One mole refers to the molecular weight of a substance expressed in grams.
Other Enthalpy Change Units
While kJ/mol is the most common unit, other units can be used to express enthalpy change, including:
| Unit | Abbreviation | Conversion to kJ/mol |
|---|---|---|
| Joule per mole | J/mol | 0.001 kJ/mol |
| Calorie per mole | cal/mol | 4.184 kJ/mol |
| Kilocalorie per mole | kcal/mol | 4.184 kJ/mol |
Converting Between Enthalpy Change Units
To convert between different enthalpy change units, use the following conversion factors:
- 1 kJ/mol = 1000 J/mol
- 1 kJ/mol = 0.239 cal/mol
- 1 kJ/mol = 0.239 kcal/mol
Significance of Enthalpy Change
Enthalpy change is a crucial parameter in chemistry as it provides valuable information about the energy involved in chemical reactions. This information is essential for:
- Predicting the direction of a reaction (exothermic or endothermic)
- Determining the spontaneity of a reaction
- Designing energy-efficient chemical processes
- Understanding reaction mechanisms
Exothermic and Endothermic Reactions
Chemical reactions can be classified based on their enthalpy change:
- Exothermic reactions: Release energy (ΔH < 0). The enthalpy of the products is lower than the enthalpy of the reactants.
- Endothermic reactions: Absorb energy (ΔH > 0). The enthalpy of the reactants is lower than the enthalpy of the products.
Table 1: Common Enthalpies of Formation
| Substance | Enthalpy of Formation (kJ/mol) |
|---|---|
| Water (liquid) | -285.8 |
| Carbon dioxide (gas) | -393.5 |
| Methane (gas) | -74.8 |
| Ethanol (liquid) | -277.7 |
| Glucose (solid) | -1273.3 |
Applications of Enthalpy Change
The concept of enthalpy change has numerous applications in chemistry and related fields:
- Thermochemistry: Quantifies the energy changes in chemical reactions.
- Chemical engineering: Designs chemical reactors and optimizes energy efficiency.
- Astrophysics: Determines the energy released in nuclear reactions in stars.
- Geochemistry: Explains the heat flow within the Earth’s crust.
- Biochemistry: Analyzes the energy metabolism in living organisms.
Table 2: Standard Enthalpies of Combustion
| Substance | Standard Enthalpy of Combustion (kJ/mol) |
|---|---|
| Methane (gas) | -890.3 |
| Propane (gas) | -2220.0 |
| Butane (gas) | -2877.8 |
| Octane (liquid) | -5471.0 |
| Ethanol (liquid) | -1367.0 |
Novel Applications of Enthalpy Change
Emerging fields are continuously exploring innovative applications of enthalpy change concepts:
- Energy storage: Enthalpy change is used to design thermal energy storage systems for renewable energy applications.
- Materials science: Enthalpy change is crucial for understanding phase transitions and material synthesis processes.
- Advanced manufacturing: Enthalpy change is utilized to optimize energy consumption in industrial processes, such as metalworking and composite fabrication.
Table 3: Enthalpies of Fusion and Vaporization
| Substance | Enthalpy of Fusion (kJ/mol) | Enthalpy of Vaporization (kJ/mol) |
|---|---|---|
| Water | 6.01 | 40.7 |
| Aluminum | 10.7 | 290 |
| Gold | 12.5 | 15.8 |
| Copper | 13.3 | 30.0 |
| Carbon dioxide | 0.76 | 18.4 |
Table 4: Enthalpies of Hydration
| Ion | Enthalpy of Hydration (kJ/mol) |
|---|---|
| H+ | -1088 |
| Na+ | -406 |
| Cl- | -363 |
| Mg2+ | -1921 |
| SO42- | -1000 |
Conclusion
Enthalpy change is a fundamental concept in chemistry that quantifies the energy exchange in chemical reactions. Its units vary, but kilojoules per mole (kJ/mol) is the most prevalent. Enthalpy change plays a vital role in predicting reaction direction, determining spontaneity, and designing chemical processes. Its applications extend beyond chemistry, finding relevance in diverse fields. As research advances, we can expect novel and inventive applications of enthalpy change concepts that further our understanding of energy and its role in shaping our world.