Introduction
Nitrogen, the seventh element on the periodic table, is the most abundant element in the Earth’s atmosphere, comprising approximately 78% of its volume. This colorless, odorless, and tasteless gas plays a crucial role in various biological processes and industrial applications. Understanding the electronic structure of nitrogen, specifically through its Lewis structure, is essential for deciphering its chemical behavior and reactivity.

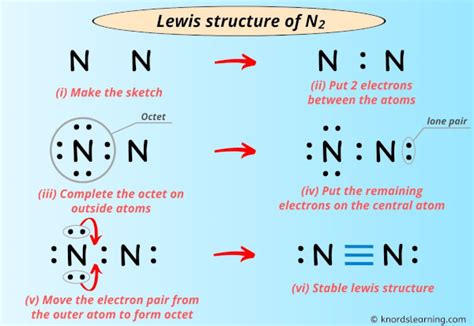
Step 1: Determine the Total Number of Valence Electrons
The Lewis structure depicts the arrangement of electrons around atoms in a molecule. To draw the Lewis structure of N2, we must first determine the total number of valence electrons involved. Nitrogen has five valence electrons, and since N2 consists of two nitrogen atoms, the total number of valence electrons becomes:
5 (for N) × 2 = 10 valence electrons
Step 2: Connect the Atoms with a Single Bond
The first step in drawing the Lewis structure is to connect the two nitrogen atoms with a single bond, representing the sharing of two electrons between them:
N—N
Step 3: Distribute the Remaining Valence Electrons
With the single bond in place, we have eight valence electrons remaining (10 total – 2 used for the bond). We distribute these electrons as lone pairs around the nitrogen atoms:
:N—N:
Each nitrogen atom now has eight valence electrons, satisfying the octet rule. The Lewis structure of N2 is complete:
:N≡N:
Properties of N2
The Lewis structure provides valuable insights into the properties of N2:
-
Triple Bond: The presence of three pairs of electrons between the nitrogen atoms indicates a triple bond, which is one of the strongest types of covalent bonds. This triple bond gives N2 its exceptional stability and inertness.
-
Linear Geometry: The electron pairs around each nitrogen atom repel each other, resulting in a linear molecular geometry. The bond angle between the nitrogen atoms is 180 degrees.
-
Low Reactivity: The strong triple bond and the inert nature of nitrogen make N2 a relatively unreactive molecule. It does not readily react with most other elements or compounds at room temperature.
Applications of N2
Despite its low reactivity, N2 finds numerous applications due to its abundance and unique properties:
-
Fertilizer Production: Nitrogen is an essential nutrient for plant growth. N2 is converted into ammonia (NH3) through the Haber-Bosch process, which is used to produce fertilizers for agriculture.
-
Refrigeration: Liquid nitrogen is widely used as a cryogenic refrigerant in scientific research and industrial processes, such as food freezing and preservation.
-
Inert Atmosphere: N2 is commonly used to create an inert atmosphere in various industrial processes, such as welding, metalworking, and chemical reactions, to prevent oxidation and contamination.
-
Medical Applications: Liquid nitrogen is used in cryosurgery to freeze and destroy abnormal tissues, such as tumors or warts. It is also used for cryopreservation, the storage of biological materials at ultra-low temperatures.
Tips for Drawing Lewis Structures
-
Count Valence Electrons: Determine the total number of valence electrons involved in the molecule.
-
Connect Atoms with Bonds: Connect the atoms with single bonds to form the molecular skeleton.
-
Distribute Remaining Electrons: Place the remaining electrons as lone pairs around the atoms to satisfy the octet rule.
-
Check Formal Charge: Ensure that the formal charge of each atom is zero, indicating that the electrons are distributed evenly.
-
Consider Resonance: In some cases, molecules may have multiple Lewis structures known as resonance structures.
Conclusion
The Lewis structure of N2 provides a clear representation of the electron distribution and bonding in this molecule. Understanding the Lewis structure is crucial for comprehending the properties and applications of N2, which is a vital element in both natural and industrial processes. By following the principles and techniques discussed in this article, one can effectively draw the Lewis structure of N2 and gain valuable insights into its chemical behavior and significance.