Understanding the Relationship Between Melting Point and Solubility
When it comes to understanding the behavior of chemical compounds, the relationship between their melting point and solubility is a crucial factor to consider. Melting point refers to the temperature at which a solid substance transitions into a liquid state, while solubility measures the extent to which a substance can dissolve in a solvent to form a homogeneous mixture.

General Correlation between Melting Point and Solubility
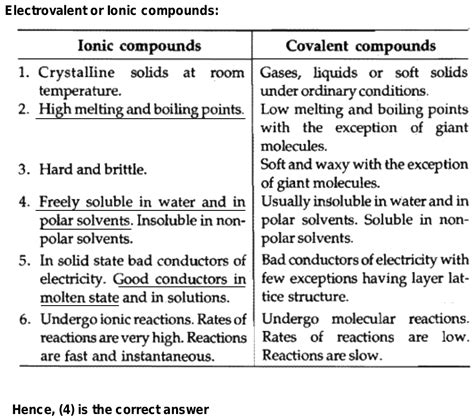
In general, there is an inverse relationship between melting point and solubility. Substances with lower melting points tend to exhibit higher solubility, while substances with higher melting points tend to have lower solubility. This relationship can be explained by the molecular structure and intermolecular forces of the substance.
How Melting Point Affects Solubility
The melting point of a substance is determined by the strength of the intermolecular forces holding the molecules together in the solid state. Strong intermolecular forces, such as covalent bonds or ionic bonds, require more energy to overcome, resulting in a higher melting point. Substances with weak intermolecular forces, such as van der Waals forces or hydrogen bonds, have lower melting points.
When a substance dissolves in a solvent, the solvent molecules interact with the solute molecules, breaking apart the intermolecular forces and allowing the solute to disperse throughout the solvent. If the intermolecular forces of the solute are weak, as is the case for substances with low melting points, it becomes easier for the solvent molecules to overcome these forces and dissolve the solute. This leads to higher solubility.
Exceptions to the General Correlation
While the inverse relationship between melting point and solubility holds true for many substances, there are some exceptions. Substances that form strong complexes with the solvent molecules may exhibit higher solubility even if they have higher melting points. For example, certain ionic compounds that interact strongly with water molecules through ion-dipole interactions can have high solubility despite having high melting points.
Practical Implications and Applications
The relationship between melting point and solubility has significant practical implications in various fields:
Pharmaceuticals:
The solubility of a drug is crucial for its bioavailability and effectiveness. Drugs with low melting points and high solubility can be more easily absorbed by the body, resulting in faster onset of action and improved efficacy.
Food Industry:
In the food industry, the solubility of ingredients is essential for product development and quality control. Substances with low melting points can enhance the texture, flavor, and stability of food products. For example, fats and oils with low melting points melt easily at room temperature, contributing to the desired smoothness and mouthfeel of various food products.
Chemical Engineering:
Chemical engineers use the solubility data of substances to design and optimize processes involving dissolution and crystallization. By understanding the relationship between melting point and solubility, they can select appropriate solvents and operating conditions to achieve desired outcomes.
Novel Applications:
The inverse relationship between melting point and solubility can inspire novel applications for substances with specific properties. For example, low-melting point materials can be used as phase-change materials for thermal energy storage and as additives in self-healing materials, where their ability to dissolve and re-form upon melting can promote repair mechanisms.
Strategies for Enhancing Solubility
Chemical Modification:
Chemical modification of a substance can alter its solubility by introducing functional groups that enhance its affinity for the solvent. For example, adding polar groups to a non-polar compound can increase its solubility in polar solvents.
Co-solvency:
Co-solvency involves using a mixture of solvents to dissolve a substance. By combining a good solvent with a poor solvent, the solubility of the substance can be significantly improved.
Temperature Manipulation:
Solubility typically increases with temperature. Heating a solution can enhance the dissolution process and increase the solubility of a substance.
Pressure Adjustment:
For gases, solubility increases with pressure. Applying pressure to a gas-liquid system can increase the amount of gas that dissolves in the liquid.
Conclusion
The relationship between melting point and solubility is a fundamental concept in chemistry with practical implications in various fields. Substances with low melting points generally exhibit higher solubility due to weaker intermolecular forces. Understanding this relationship is crucial for optimizing processes involving dissolution, crystallization, and the development of innovative applications for substances with specific properties.