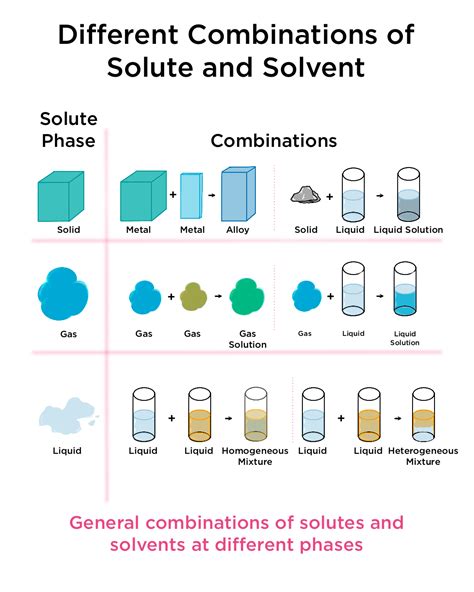
Understanding Dissolution Chemistry Equation
Dissolution chemistry equation is a chemical equation that describes the process of a solute dissolving in a solvent. The general form of the equation is:

Solute + Solvent → Solution
where:
- Solute is the substance that is being dissolved.
- Solvent is the substance that the solute is dissolved in.
- Solution is the homogeneous mixture that results from the dissolution process.
The dissolution process is a dynamic equilibrium, meaning that the solute and solvent are constantly interacting with each other. The rate at which the solute dissolves depends on several factors, including the temperature, pressure, and the nature of the solute and solvent.
Key Concepts in Dissolution Chemistry Equation
Several key concepts are involved in dissolution chemistry equation, including:
Solubility
Solubility is the maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature and pressure. The solubility of a solute is determined by its chemical structure, the nature of the solvent, and the temperature and pressure of the system.
Dissolution Rate
The dissolution rate is the rate at which the solute dissolves in the solvent. The dissolution rate is determined by several factors, including the temperature, pressure, the surface area of the solute, and the agitation of the solution.
Equilibrium Constant
The equilibrium constant is a measure of the extent to which a dissolution reaction proceeds. The equilibrium constant is determined by the temperature and pressure of the system.
Applications of Dissolution Chemistry Equation
Dissolution chemistry equation is used in a wide variety of applications, including:
- Pharmaceuticals: Dissolution chemistry equation is used to design drug delivery systems that control the release of drugs into the body.
- Food science: Dissolution chemistry equation is used to develop new food products and to improve the quality of existing food products.
- Environmental science: Dissolution chemistry equation is used to study the fate of pollutants in the environment.
- Industrial chemistry: Dissolution chemistry equation is used to design and optimize chemical processes.
Importance of Dissolution Chemistry Equation
Dissolution chemistry equation is important because it provides a framework for understanding the dissolution process. The dissolution chemistry equation can be used to predict the solubility of a solute, the dissolution rate, and the equilibrium constant. This information can be used to design and optimize a wide variety of applications.
Strategies for Enhancing Dissolution
Several strategies can be used to enhance the dissolution of a solute. These strategies include:
- Increasing the temperature: Increasing the temperature increases the kinetic energy of the solute molecules, which leads to a higher dissolution rate.
- Decreasing the pressure: Decreasing the pressure decreases the concentration of the solute in the solution, which leads to a higher dissolution rate.
- Increasing the surface area of the solute: Increasing the surface area of the solute increases the number of solute molecules that are in contact with the solvent, which leads to a higher dissolution rate.
- Adding a surfactant: A surfactant is a substance that reduces the surface tension of the solvent. This can lead to a higher dissolution rate by increasing the contact between the solute and the solvent.
Benefits of Enhancing Dissolution
Enhancing the dissolution of a solute can provide several benefits. These benefits include:
- Improved bioavailability: Improved bioavailability means that more of the solute is available to the body. This can be important for drugs, nutrients, and other substances that need to be absorbed into the body.
- Faster reaction rates: Faster reaction rates can lead to increased productivity and efficiency in chemical processes.
- Improved product quality: Improved product quality can be achieved by controlling the dissolution of solutes in food products, pharmaceuticals, and other products.
FAQs
1. What is the difference between dissolution and solubility?
Dissolution is the process of a solute dissolving in a solvent. Solubility is the maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature and pressure.
2. What factors affect the dissolution rate?
The dissolution rate is affected by several factors, including the temperature, pressure, the surface area of the solute, and the agitation of the solution.
3. How can the dissolution of a solute be improved?
The dissolution of a solute can be improved by increasing the temperature, decreasing the pressure, increasing the surface area of the solute, and adding a surfactant.
4. What are the benefits of enhancing dissolution?
Enhancing the dissolution of a solute can provide several benefits, including improved bioavailability, faster reaction rates, and improved product quality.
5. What is the dissolution chemistry equation?
The dissolution chemistry equation is a chemical equation that describes the process of a solute dissolving in a solvent. The general form of the equation is:
Solute + Solvent → Solution
6. What are some applications of the dissolution chemistry equation?
The dissolution chemistry equation is used in a wide variety of applications, including pharmaceuticals, food science, environmental science, and industrial chemistry.
7. What are some factors that affect the rate of dissolution?
The rate of dissolution is affected by the temperature, the surface area of the solute, the concentration of the solute, and the agitation of the solution.
8. What are some ways to increase the rate of dissolution?
The rate of dissolution can be increased by increasing the temperature, increasing the surface area of the solute, decreasing the concentration of the solute, and agitating the solution.