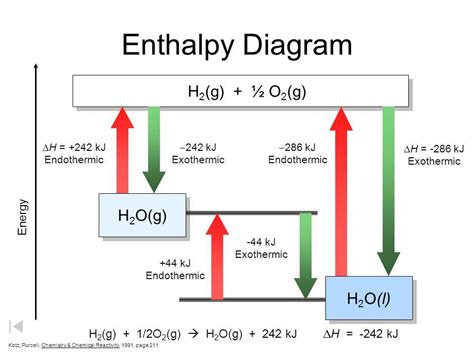
Introduction

Enthalpy, a fundamental thermodynamic property, plays a critical role in understanding various physical and chemical processes. Its units are essential for accurate measurements and calculations, ensuring consistency and reliability in scientific investigations. This article delves into the intricacies of enthalpy units, exploring their significance and providing practical guidance for their effective use.
SI Units of Enthalpy
The International System of Units (SI) defines the unit of enthalpy as the joule (J). It represents the total energy of a system, including its internal thermal energy and the work done by or on the system due to changes in its volume.
Common Units
While joules are the primary SI unit for enthalpy, other commonly used units include:
- kilojoules (kJ): 1 kJ = 1000 J
- calorie (cal): 1 cal = 4.184 J
- kilocalorie (kcal): 1 kcal = 1000 cal = 4184 J
Conversion Factors
Converting between different enthalpy units is crucial for comparing experimental data and ensuring accuracy. The following conversion factors are essential:
Table 1: Enthalpy Conversion Factors
| Unit | Conversion Factor to Joules |
|---|---|
| kilojoule (kJ) | 1 kJ = 1000 J |
| calorie (cal) | 1 cal = 4.184 J |
| kilocalorie (kcal) | 1 kcal = 4184 J |
Importance of Units
Using the correct enthalpy units is essential for several reasons:
- Consistency: It ensures the uniformity of measurements across scientific studies and disciplines.
- Accuracy: Accurate unit conversion prevents errors in calculations and ensures reliable results.
- Comparability: It allows for meaningful comparisons between data obtained using different measurement techniques.
- Communication: Clearly stating the units facilitates effective communication and understanding among researchers.
Selecting the appropriate enthalpy units depends on the specific context and the magnitude of the energy changes being investigated.
- Small-scale processes: For small-scale experiments or calculations, such as in laboratory settings, millijoules (mJ) or kilojoules (kJ) are commonly used.
- Large-scale processes: In industrial applications or large-scale chemical reactions, megajoules (MJ) or gigajoules (GJ) may be more appropriate.
- Biological systems: In biochemistry and physiology, kilocalories (kcal) are often used to measure energy changes in living organisms.
Understanding enthalpy units is crucial for a wide range of applications, including:
- Thermodynamics: Enthalpy changes are used to determine the spontaneity and efficiency of chemical reactions.
- Chemical reactions: Enthalpy measurements provide insights into the energy released or absorbed during chemical transformations.
- Heat transfer: Enthalpy changes are essential for calculating heat flow and temperature changes in various systems.
- Material science: Enthalpy changes play a role in understanding material properties, phase transitions, and thermal stability.
- Biological processes: Enthalpy changes are involved in metabolic reactions, nutrient breakdown, and energy utilization within living organisms.
Several common mistakes can arise when working with enthalpy units:
- Using incorrect conversion factors: Double-checking conversion factors is crucial to prevent inaccuracies.
- Mixing different units: Avoid mixing different enthalpy units within the same calculation or equation.
- Ignoring the sign of enthalpy: Enthalpy changes can be positive (exothermic) or negative (endothermic); ignoring the sign can lead to incorrect conclusions.
- Assuming all enthalpies are at constant temperature: Enthalpy changes are typically measured at constant pressure; assuming constant temperature may not always be appropriate.
- Identify the context: Determine the scale and nature of the process being investigated to choose suitable enthalpy units.
- Choose the SI unit: Use joules (J) as the primary unit of enthalpy whenever possible to ensure consistency.
- Convert to appropriate units: If necessary, convert the enthalpy value to the desired unit using the appropriate conversion factor.
- Check the sign: Ensure the sign of the enthalpy change (+ or -) is correctly represented.
- State the units clearly: Always clearly indicate the enthalpy units used in calculations and measurements.
To further enhance the understanding and exploration of enthalpy units, we propose introducing the term “enthalpy engineering”, which encompasses the innovative and systematic manipulation of enthalpy changes to achieve desired outcomes. This concept could lead to advancements in energy-efficient processes, material design, and biomedical applications.
Tables
Table 2: Enthalpy Values for Common Substances
| Substance | Enthalpy of Formation (kJ/mol) |
|---|---|
| Water (H2O, liquid) | -285.8 |
| Carbon dioxide (CO2, gas) | -393.5 |
| Ethanol (C2H5OH, liquid) | -277.6 |
| Methane (CH4, gas) | -74.8 |
| Glucose (C6H12O6, solid) | -1264.9 |
Table 3: Enthalpy Changes in Biological Processes
| Process | Enthalpy Change (kcal/mol) |
|---|---|
| Cellular respiration | -686 |
| Photosynthesis | 114 |
| ATP hydrolysis | -7.3 |
| Protein synthesis | 20-30 |
Table 4: Enthalpy Applications in Various Fields
| Field | Application |
|---|---|
| Thermodynamics | Calculating heat flow, efficiency of processes |
| Chemical reactions | Predicting reaction spontaneity, determining energy released/absorbed |
| Material science | Understanding phase transitions, thermal stability |
| Biology | Investigating metabolic reactions, energy utilization |
| Engineering | Designing energy-efficient systems, optimizing processes |
Conclusion
Understanding the units of enthalpy is crucial for accuracy, consistency, and effective communication in scientific endeavors. By carefully considering the context, selecting appropriate units, and avoiding common mistakes, researchers can ensure the validity and reliability of their measurements and insights. The concept of “enthalpy engineering” presents exciting opportunities for future innovations and discoveries.