Understanding Molar Solubility
Molar solubility, expressed in moles per liter (mol/L), quantifies the maximum concentration of a solute that can dissolve in a given solvent at a specific temperature and pressure. This concept plays a crucial role in various chemical processes, including crystallization, precipitation, and analytical chemistry.

Factors Influencing Molar Solubility
Numerous factors influence the molar solubility of a solute in a solvent:
- Nature of the Solvent and Solute: Polar solvents dissolve polar solutes, while non-polar solvents dissolve non-polar solutes. The stronger the polarity difference, the higher the solubility.
- Temperature: Generally, the molar solubility of a solid solute in a liquid solvent increases with increasing temperature. This is because elevated temperatures provide more kinetic energy, promoting solute dissolution.
- Pressure: In the case of gases dissolved in liquids, the molar solubility is directly proportional to the partial pressure of the gas above the liquid.
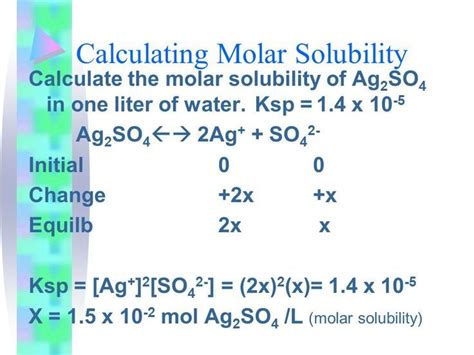
Determining Molar Solubility
The most common method for determining molar solubility involves an equilibrium approach. Consider a saturated solution of a solid solute in a liquid solvent. At equilibrium:
Solid Solute (s) ⇌ Ionized Solute (aq)
The equilibrium constant, Ksp (solubility product constant), is a measure of the solubility of the solute. Ksp is expressed as the product of the molar concentrations of the ionized solute ions raised to their stoichiometric coefficients.
For example, consider the dissolution of calcium carbonate (CaCO3) in water:
CaCO3 (s) ⇌ Ca2+ (aq) + CO32- (aq)
The Ksp for CaCO3 is 4.87 x 10^-9. At equilibrium, the molar solubility of CaCO3 can be calculated as:
Molar Solubility = [Ca2+] = [CO32-] = (Ksp/4)^(1/2) = 2.21 x 10^-5 mol/L
Practical Applications
Calculating molar solubility has numerous practical applications, including:
- Solubility Prediction: Determining the maximum concentration of a solute that can dissolve in a given solvent at a given temperature and pressure.
- Solubility Enhancement: Designing strategies to increase the solubility of sparingly soluble drugs or industrial chemicals.
- Precipitation Control: Predicting the conditions under which a solute will precipitate from a solution, preventing unwanted crystallization or clogging.
- Environmental Monitoring: Assessing the solubility of pollutants in water or soil to determine their potential impact on ecosystems.
Advanced Applications
Beyond traditional solubility calculations, new applications are emerging in various fields:
- Nanotechnology: Controlling the solubility of nanomaterials for targeted delivery and release.
- Green Chemistry: Developing environmentally friendly solvents and synthetic strategies to improve solubility and reduce waste.
- Biotechnology: Engineering proteins and antibodies with enhanced solubility for therapeutic or diagnostic applications.
Tips and Tricks
- Use reliable sources, such as chemical handbooks or peer-reviewed literature, for accurate Ksp values.
- Consider the effects of common ion effects, which can significantly decrease solubility.
- Utilize solubility tables or online calculators to simplify calculations.
- Experimentally determine solubility using gravimetric or spectrophotometric methods for non-standard conditions.
Pros and Cons of Molar Solubility Calculations
Pros:
- Provides quantitative information about the maximum concentration of a solute that can dissolve in a solvent.
- Allows for the prediction and control of solubility in various applications.
- Supports the understanding of chemical equilibria and solution behavior.
Cons:
- May not always accurately represent solubility in complex or non-ideal solutions.
- Requires knowledge of the Ksp for the specific solute-solvent system.
- Can be challenging to calculate for solutes with low solubility.
FAQs
-
What is the difference between solubility and molar solubility?
Solubility refers to the qualitative ability of a solute to dissolve in a solvent, while molar solubility quantifies the maximum concentration of the solute in a saturated solution. -
How does temperature affect molar solubility?
Generally, molar solubility increases with increasing temperature for solid solutes in liquids. -
What is the Ksp for a substance?
The Ksp is the equilibrium constant that describes the solubility of a compound in a given solvent at a specific temperature. -
How can molar solubility be used to predict precipitation?
If the ion product (IP) of a solution exceeds the Ksp, precipitation is likely to occur. -
What are the limitations of molar solubility calculations?
They may not be accurate for complex solutions or for solutes with low solubility. -
How can molar solubility be experimentally determined?
Gravimetric or spectrophotometric methods can be used to determine the concentration of a solute in a saturated solution. -
What are some applications of molar solubility in industry?
Solubility calculations are used in pharmaceutical, chemical, and environmental industries for product design, quality control, and pollution monitoring. -
How can molar solubility be improved?
Techniques such as adding surfactants, using polar solvents, or applying high pressure can enhance solubility.