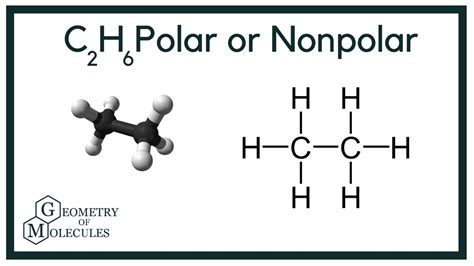
In the realm of chemistry, polarity plays a crucial role in understanding the behavior and properties of molecules. Ethane, a simple yet ubiquitous hydrocarbon, sparks curiosity among researchers and students alike, raising the question: Is C2H6 polar or nonpolar? Embark on a comprehensive exploration of this topic, delving into the fundamental concepts, exploring the scientific evidence, and uncovering the multifaceted applications of ethane.

Understanding Polarity: A Balancing Act
Polarity refers to the uneven distribution of electrical charge within a molecule. It arises when the electronegativity of atoms within a molecule differs, causing electrons to be unequally shared. Electronegativity, a measure of an atom’s ability to attract electrons, determines the polarity of covalent bonds.
Ethane: A Nonpolar Molecule
Ethane (C2H6), consisting of two carbon atoms bonded to six hydrogen atoms, exhibits a nonpolar nature. This nonpolarity stems from the equal sharing of electrons between the carbon and hydrogen atoms. The electronegativity of carbon and hydrogen are relatively close (2.55 and 2.20, respectively), resulting in a balanced distribution of electrical charge. Consequently, ethane exhibits no net dipole moment, rendering it a nonpolar molecule.
Applications of Nonpolar Ethane
Nonpolarity endows ethane with unique properties that find applications in various industries:
-
Fuel: Ethane serves as a major component of natural gas, providing a clean and efficient fuel source for homes, industries, and power plants.
-
Petrochemicals: As a versatile feedstock, ethane is used to produce a plethora of petrochemicals such as ethylene, polyethylene, and vinyl chloride, which form the backbone of plastics, packaging, and other synthetic materials.
-
Refrigerant: Given its nonpolar and low-boiling nature, ethane can be used as a refrigerant in refrigeration systems.
-
Aerosols: Ethane is employed as a propellant in aerosol cans, dispensing a variety of products ranging from personal care items to household cleaners.
Tables: Illuminating Key Data
| Property | Value |
|---|---|
| Molecular Weight | 30.07 g/mol |
| Melting Point | -183.3 °C |
| Boiling Point | -88.6 °C |
| Density (at 25 °C) | 0.546 g/mL |
| Applications | Examples |
|---|---|
| Fuel | Natural gas, Liquefied Natural Gas (LNG) |
| Petrochemicals | Ethylene, polyethylene, vinyl chloride |
| Refrigerant | Refrigerators, air conditioners |
| Aerosols | Hairsprays, deodorants, cleaning sprays |
Tips and Tricks for Understanding Polarity
-
Electronegativity Table: A handy reference, the electronegativity table provides the electronegativity values of elements, aiding in predicting polarity.
-
Molecular Geometry: Understand the three-dimensional shape of molecules to determine their polarity. Symmetrical molecules often display nonpolarity, while asymmetrical molecules tend to be polar.
-
Dipole Moment: Calculate the dipole moment of a molecule to quantify its polarity. A non-zero dipole moment indicates a polar molecule.
Conclusion
The nonpolar nature of ethane, resulting from the balanced electron distribution between carbon and hydrogen atoms, plays a vital role in its properties and applications. As a clean-burning fuel, versatile feedstock for petrochemicals, and non-toxic refrigerant, ethane remains a valuable substance in the chemical industry. By understanding the concept of polarity and its implications in ethane, researchers and practitioners can unlock innovative applications and optimize the use of this ubiquitous hydrocarbon.