Introduction

The Lewis dot structure is a powerful tool in chemistry that provides a visual representation of a molecule’s electron arrangement. For carbon tetrachloride (C2Cl4), understanding its Lewis dot structure is crucial for comprehending its chemical properties and reactivity.
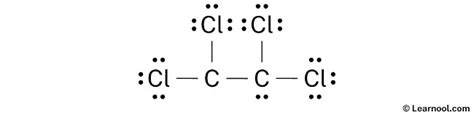
Lewis Dot Structure of C2Cl4
Carbon tetrachloride’s Lewis dot structure depicts the distribution of valence electrons in the molecule. Each chlorine atom has seven valence electrons, while each carbon atom has four. The structure is drawn as follows:
:Cl: :Cl:
\ / /
C - C \ /
/ \ /
:Cl: :Cl:
Key Features of the Lewis Dot Structure
-
Tetrahedral Shape: The carbon atoms in C2Cl4 are sp3 hybridized, resulting in a tetrahedral electron geometry and molecular shape. This means the chlorine atoms are arranged in a pyramid-like structure around the carbon atoms.
-
Nonpolar Molecule: The electronegativity of carbon and chlorine is similar. As a result, the electrons are shared equally between the atoms, creating a nonpolar molecule.
-
Zero Net Charge: The total number of valence electrons in the molecule is 32 (4 from each carbon and 7 from each chlorine). Since the number of electrons is equal to the number of protons, C2Cl4 has a net charge of zero.
Applications of C2Cl4
C2Cl4 was once used extensively as a solvent, dry-cleaning agent, and refrigerant due to its nonflammability and nonpolarity. However, its use has declined due to its environmental and health hazards.
Environmental Impact: C2Cl4 is a volatile organic compound (VOC) that contributes to air pollution and climate change. It is also a persistent organic pollutant (POP) that bioaccumulates in the environment, posing risks to wildlife and human health.
Health Hazards: C2Cl4 is toxic to the liver, kidneys, and nervous system. It can cause skin irritation, respiratory problems, and even liver cancer with prolonged exposure.
Novel Applications
Despite its environmental and health concerns, C2Cl4 has shown promise in certain niche applications:
-
Synthesis of Pharmaceuticals: C2Cl4 is used as a building block for synthesizing various pharmaceutical drugs.
-
Advanced Materials: C2Cl4 can be utilized in the production of carbon fibers and other advanced materials.
-
Alternative Fuels: Research is exploring the potential of C2Cl4 as an alternative fuel in the transportation sector.
Tables
Table 1: Key Properties of C2Cl4
| Property | Value |
|---|---|
| Molecular Weight | 153.82 g/mol |
| Melting Point | -23 °C |
| Boiling Point | 76.5 °C |
| Density | 1.59 g/cm³ |
Table 2: Toxicity of C2Cl4
| Organ | Effect |
|---|---|
| Liver | Liver damage, cirrhosis |
| Kidneys | Kidney damage, failure |
| Nervous System | Neurological disorders, seizures |
| Skin | Skin irritation, chemical burns |
Table 3: Applications of C2Cl4
| Application | Description |
|---|---|
| Solvent | Nonpolar solvent for various applications |
| Dry-Cleaning Agent | Solvent for cleaning fabrics and textiles |
| Refrigerant | Refrigerant in refrigeration systems |
| Pharmaceutical Synthesis | Building block for drug synthesis |
| Advanced Materials | Precursor for carbon fibers and other materials |
Table 4: Alternative Applications of C2Cl4
| Application | Description |
|---|---|
| Fuel Additive | Potential alternative fuel for transportation |
| Pesticide | Insecticide for specific agricultural applications |
| Industrial Cleaning | Solvent for cleaning machinery and industrial equipment |
Tips and Tricks
- When drawing the Lewis dot structure of C2Cl4, remember that each carbon atom forms four bonds, while each chlorine atom forms one bond.
- Use a periodic table to check the valence electron configuration of each element.
- Double and triple bonds are not possible between carbon and chlorine in C2Cl4 because carbon has already formed four single bonds in the tetrahedral structure.
- C2Cl4 is a nonpolar molecule, which means it will not dissolve in polar solvents like water.
Common Mistakes to Avoid
- Do not assign an incorrect number of valence electrons to each atom.
- Do not form double or triple bonds between carbon and chlorine.
- Do not assume that C2Cl4 is polar due to the presence of chlorine atoms.
- Do not underestimate the environmental and health hazards associated with C2Cl4.
FAQs
Q1: What is the molecular shape of C2Cl4?
A1: Tetrahedral
Q2: Is C2Cl4 a polar molecule?
A2: No, it is nonpolar
Q3: What are the health hazards of C2Cl4?
A3: Liver damage, kidney damage, nervous system disorders, and skin irritation
Q4: What are some alternative applications for C2Cl4?
A4: Fuel additive, pesticide, industrial cleaning solvent
Q5: Why is C2Cl4 no longer widely used as a solvent or refrigerant?
A5: Due to its environmental and health hazards
Q6: What is the significance of C2Cl4 in drug synthesis?
A6: It serves as a building block for various pharmaceutical drugs
Q7: Can C2Cl4 be used as a fuel?
A7: Research is ongoing to explore its potential as an alternative fuel
Q8: What should be considered when handling C2Cl4?
A8: Wear appropriate protective gear, avoid prolonged exposure, and handle in a well-ventilated area