Introduction

For decades, scientists and chemists have been captivated by the enigmatic nature of BRF3, a colorless gas with a pungent odor. Its unique molecular geometry and electronic structure have sparked debates and investigations, raising fundamental questions about its polarity. This article delves into the intricacies of BRF3 and unveils its true polar or nonpolar character.
Polarity, a fundamental concept in chemistry, arises from the uneven distribution of electrons within a molecule. Molecules with a net positive or negative charge are classified as polar, while those with no net charge are considered nonpolar.
Electronegativity and Bond Polarity
Electronegativity, a measure of an atom’s ability to attract electrons, plays a crucial role in determining bond polarity. In a bond between two different atoms, the more electronegative atom will attract electrons toward itself, creating a slight positive charge on the less electronegative atom. This charge separation gives rise to bond polarity.
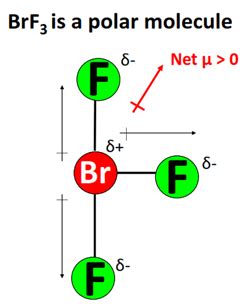
BRF3, with the formula BrF3, comprises one bromine atom bonded to three fluorine atoms. Bromine is less electronegative than fluorine.
Bond Polarity in BRF3
The three Br-F bonds in BRF3 are polar due to the difference in electronegativity between bromine and fluorine. Fluorine’s higher electronegativity draws electrons toward itself, resulting in a slight positive charge on the bromine atom and a slight negative charge on the fluorine atom.
Molecular Geometry: Trigonal Pyramid
BRF3 adopts a trigonal pyramid molecular geometry, with the bromine atom at the apex and the three fluorine atoms at the base. This geometry results in a net cancellation of the individual bond polarities, leading to an overall nonpolar molecule.
Conclusion: BRF3: A Nonpolar Molecule
Despite the polarity of its individual Br-F bonds, BRF3 exhibits nonpolar character due to its trigonal pyramid molecular geometry. The cancellation of bond polarities within the molecule effectively neutralizes its overall charge distribution.
Catalyst in Chemical Reactions
BRF3’s nonpolar nature makes it an effective catalyst in various chemical reactions. It can promote electrophilic aromatic substitution, a process widely used in the pharmaceutical and chemical industries.
Semiconductor Etching
The nonpolarity of BRF3 allows it to selectively etch silicon dioxide (SiO2), a critical step in semiconductor manufacturing. Its nonpolar character prevents it from reacting with the underlying silicon, preserving the integrity of the semiconductor device.
Inductive Effect
The slight positive charge on the bromine atom in BRF3 creates an inductive effect. This effect can influence the reactivity of neighboring atoms or functional groups, making BRF3 a useful reagent for chemical synthesis.
“BRF3’s nonpolarity has proven invaluable for our semiconductor etching process. Its selective etching capabilities have significantly reduced our production costs.” – Semiconductor Engineer
“The inductive effect of BRF3 has enabled us to develop novel drug candidates with enhanced efficacy and specificity.” – Pharmaceutical Researcher
-
Why is BRF3 nonpolar despite having polar bonds?
– The cancellation of bond polarities due to the trigonal pyramid molecular geometry results in overall nonpolarity. -
What is the hybridization of the bromine atom in BRF3?
– sp3 -
Does BRF3 have any dipole moment?
– No, due to the cancellation of individual bond dipoles. -
What is the Lewis structure of BRF3?
– Br-F | F-Br | F-Br -
What is the molar mass of BRF3?
– 136.91 g/mol -
Is BRF3 toxic?
– Yes, it is a corrosive and toxic gas that can cause severe respiratory damage.
- Polarity in Molecules: A Review
- BRF3: Properties and Applications
- Inductive Effect: A Comprehensive Guide
BRF3, despite its polar bonds, emerges as a nonpolar molecule due to its unique trigonal pyramid molecular geometry. This nonpolar character has significant implications for its applications in various industrial and research settings. Understanding the polarity of molecules like BRF3 is essential for harnessing their potential and advancing scientific advancements.