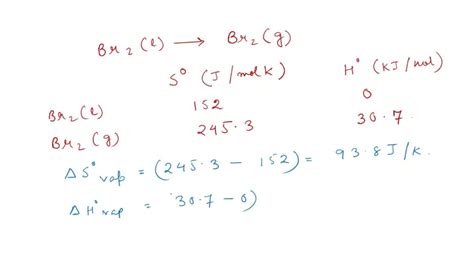Introduction
Bromine (Br2), a diatomic molecule, possesses unique physicochemical properties, including a distinct boiling point that plays a significant role in its various applications. Understanding the boiling point of Br2 is essential for advancements in chemical synthesis, industrial processes, and biomedical research. This article delves into the scientific underpinnings of Br2 boiling point, exploring its implications and practical applications.

Boiling Point of Br2
Boiling point, a fundamental property of substances, represents the temperature at which the vapor pressure of a liquid equals the pressure surrounding it, causing a phase transition to a gas. The boiling point of Br2 under standard atmospheric pressure (1 atm) has been meticulously determined to be 58.8°C (137.8°F). This relatively high boiling point is attributed to the strong intermolecular forces present between Br2 molecules.
Intermolecular Forces and Boiling Point
Intermolecular forces, including van der Waals forces and dipole-dipole interactions, govern the cohesion between molecules and influence their boiling points. In the case of Br2, the van der Waals forces arise from the temporary fluctuations in electron distribution, creating transient dipoles that interact with neighboring molecules. These forces contribute significantly to the cohesive energy of Br2, requiring a higher temperature to overcome them and enable vaporization.
Impact on Chemical Reactivity
The high boiling point of Br2 has a profound impact on its chemical reactivity. At temperatures below its boiling point, Br2 exists primarily as a liquid, facilitating its use as a solvent and a reagent in various chemical reactions. However, upon heating beyond its boiling point, Br2 transitions into the gas phase, significantly reducing its reactivity. This behavior is particularly relevant in industrial processes where temperature control is crucial for maintaining optimal reaction conditions.
Applications of Br2 Boiling Point
The boiling point of Br2 plays a vital role in a diverse range of applications:
-
Chemical Synthesis: Br2 is utilized in the synthesis of various organic compounds, including brominated hydrocarbons, pharmaceuticals, and agrochemicals. Its high boiling point enables the precise control of reaction conditions, ensuring efficient product formation.
-
Industrial Processes: In industries like metal refining and water treatment, Br2 is employed as a disinfectant and oxidant. Its high boiling point allows for safe and effective application in these processes, preventing premature vaporization and ensuring optimal efficacy.
-
Biomedical Research: Br2 has gained attention in biomedical research due to its bacteriostatic and antiviral properties. Its high boiling point facilitates the development of topical formulations, such as skin disinfectants and antimicrobial coatings, where controlled release and prolonged activity are desired.
Common Mistakes to Avoid
When working with Br2, it is important to avoid common mistakes that can compromise safety and accuracy:
-
Improper Handling: Br2 is a corrosive and toxic substance. Proper precautions, including the use of personal protective equipment and proper ventilation, must be taken when handling it.
-
Incorrect Temperature Control: Failure to maintain precise temperature control during chemical reactions involving Br2 can lead to uncontrolled vaporization, affecting reaction outcomes.
Why Br2 Boiling Point Matters
Understanding the boiling point of Br2 is essential for several reasons:
-
Safety: The high boiling point of Br2 ensures that it remains in liquid form under normal atmospheric conditions, reducing the risk of exposure and inhalation hazards.
-
Efficiency: Controlling the boiling point allows for optimized chemical reactions and industrial processes, enhancing efficiency and yield.
-
Innovation: The unique boiling point of Br2 creates opportunities for novel applications, particularly in the realm of biomedical research and drug discovery.
Innovative Applications
The high boiling point of Br2 has inspired creative new applications, including:
-
Vapor-Phase Sterilization: The development of vapor-phase sterilization techniques utilizing Br2, leveraging its high boiling point and disinfectant properties to effectively sterilize medical devices and sensitive materials.
-
Antimicrobial Coatings: The creation of antimicrobial coatings infused with Br2, exploiting its sustained release properties due to its high boiling point, providing long-term protection against microbial contamination.
-
Biomedical Theranostics: The exploration of Br2 derivatives for biomedical theranostics, combining diagnostic and therapeutic capabilities, utilizing the high boiling point to control drug delivery and enhance efficacy.
Tables
Table 1: Physicochemical Properties of Br2
| Property | Value |
|---|---|
| Boiling Point (1 atm) | 58.8°C (137.8°F) |
| Melting Point | -7.3°C (18.9°F) |
| Density (25°C) | 3.12 g/mL |
| Vapor Pressure (25°C) | 178 mmHg |
Table 2: Applications of Br2 Boiling Point
| Application | Boiling Point Significance |
|---|---|
| Chemical Synthesis | Precise control of reaction conditions |
| Industrial Processes | Safe and effective application as a disinfectant and oxidant |
| Biomedical Research | Controlled release and prolonged activity in topical formulations |
Table 3: Intermolecular Forces Influencing Br2 Boiling Point
| Intermolecular Force | Description |
|---|---|
| Van der Waals Forces | Temporary fluctuations in electron distribution, creating transient dipoles |
| Dipole-Dipole Interactions | Interactions between permanent dipoles present on molecules |
Table 4: Safety Considerations for Br2 Handling
| Potential Hazard | Precautionary Measures |
|---|---|
| Corrosivity | Wear personal protective equipment, such as gloves and goggles |
| Toxicity | Ensure adequate ventilation and avoid direct inhalation |
| Flammability | Keep away from heat sources and open flames |
Conclusion
The boiling point of Br2, a crucial physical property, underpins its diverse applications in chemical synthesis, industrial processes, and biomedical research. Its high boiling point stems from strong intermolecular forces, influencing its reactivity and contributing to its unique properties. By understanding the science behind Br2 boiling point, industries can optimize existing applications and innovate new ones, while researchers can explore novel avenues in drug discovery and therapeutic interventions. Continued research and development will undoubtedly lead to further breakthroughs and advancements in the utilization of this versatile substance.