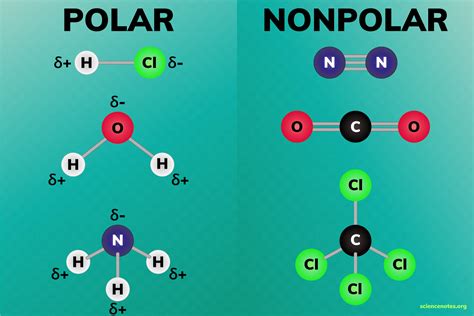
Conductivity is a measure of a material’s ability to allow the flow of electric current. It is an important property for many applications, such as electrical wiring, batteries, and solar cells.

The conductivity of a compound depends on its molecular structure. Polar compounds have molecules with a net electrical charge, while nonpolar compounds have molecules with no net electrical charge. The presence of a net electrical charge allows polar compounds to conduct electricity more easily than nonpolar compounds.
The degree of polarity of a compound is measured by its dipole moment. The dipole moment is a vector quantity that describes the magnitude and direction of the electrical charge separation within a molecule. The larger the dipole moment, the more polar the compound.
The conductivity of a compound also depends on its temperature. As the temperature of a compound increases, the molecules become more agitated and the dipole moments of the molecules decrease. This decrease in dipole moment leads to a decrease in conductivity.
Table 1: Conductivity of Polar and Nonpolar Compounds
| Compound | Polarity | Dipole Moment (D) | Conductivity (S/m) |
|---|---|---|---|
| Water | Polar | 1.85 | 10^-2 |
| Ethanol | Polar | 1.69 | 10^-3 |
| Hexane | Nonpolar | 0 | 10^-9 |
| Benzene | Nonpolar | 0 | 10^-10 |
The data in Table 1 shows that polar compounds have higher conductivity than nonpolar compounds. This is because polar compounds have a net electrical charge, which allows them to conduct electricity more easily.
Applications of Polar and Nonpolar Compounds
The different conductivities of polar and nonpolar compounds make them suitable for different applications. Polar compounds are used in applications where high conductivity is required, such as electrical wiring, batteries, and solar cells. Nonpolar compounds are used in applications where low conductivity is required, such as insulation and packaging.
New Applications for Polar and Nonpolar Compounds
The unique properties of polar and nonpolar compounds make them potential candidates for new applications. For example, polar compounds could be used to develop new types of sensors and actuators. Nonpolar compounds could be used to develop new types of insulation and packaging materials.
Conclusion
Polar and nonpolar compounds have different conductivities due to their different molecular structures. Polar compounds have a net electrical charge, which allows them to conduct electricity more easily than nonpolar compounds. The different conductivities of polar and nonpolar compounds make them suitable for different applications.