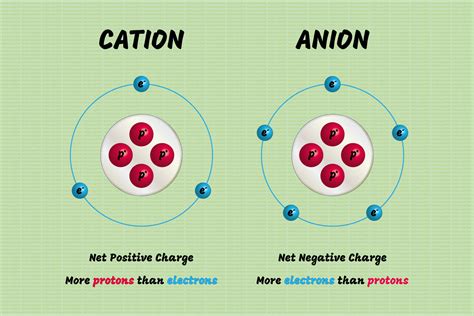
Covalent bonds, the foundation of molecular chemistry, are characterized by the sharing of electron pairs between atoms. This unique bonding mechanism contrasts with ionic bonds, where electrons are transferred from one atom to another. Therefore, covalent bonds do not form cations or anions, as the atoms involved do not have a net electrical charge.

Understanding Covalent Bonding
In a covalent bond, two atoms contribute one electron each to form a shared electron pair. This shared pair is attracted to the nuclei of both atoms, holding them together. The strength of a covalent bond is determined by the number of shared electron pairs and the electronegativity of the atoms involved.
Key Characteristics of Covalent Bonds
- Electron Sharing: Covalent bonds involve the sharing of electron pairs, not the transfer of electrons.
- Neutral Charge: The atoms involved in a covalent bond have no net electrical charge, unlike ions in ionic bonds.
- Strong Bonding: Covalent bonds are typically stronger than ionic bonds due to the sharing of electron pairs.
- Molecular Formation: Covalent bonds enable atoms to form molecules by combining their atomic orbitals.
Examples of Covalent Bonds
Covalent bonds are ubiquitous in organic chemistry and are found in a wide range of molecules, including:
- Water (H2O): Two hydrogen atoms covalently bond to an oxygen atom.
- Methane (CH4): A carbon atom covalently bonds to four hydrogen atoms.
- Carbon dioxide (CO2): A carbon atom covalently bonds to two oxygen atoms.
Applications of Covalent Bonding
The concept of covalent bonding has revolutionized chemistry and has countless applications across various fields:
- Pharmaceuticals: Drugs are designed to interact with target molecules through covalent bonds.
- Materials Science: Covalent bonding enables the synthesis of new materials with tailored properties.
- Biochemistry: Covalent bonds play a crucial role in biological processes such as DNA replication and protein synthesis.
Tables of Key Information
Table 1: Comparison of Covalent and Ionic Bonds
| Property | Covalent Bond | Ionic Bond |
|---|---|---|
| Nature of the bond | Sharing of electron pairs | Transfer of electrons |
| Net charge of atoms | Neutral | Positive (cation) or negative (anion) |
| Bond strength | Typically stronger | Typically weaker |
Table 2: Examples of Covalent and Ionic Compounds
| Covalent Compound | Ionic Compound |
|---|---|
| Water (H2O) | Sodium chloride (NaCl) |
| Methane (CH4) | Calcium oxide (CaO) |
| Carbon dioxide (CO2) | Potassium fluoride (KF) |
Table 3: Applications of Covalent Bonding in Different Fields
| Field | Application |
|---|---|
| Pharmaceuticals | Drug design and synthesis |
| Materials Science | Nanomaterial synthesis and functionalization |
| Biochemistry | DNA sequencing and genetic engineering |
| Electronics | Semiconductor manufacturing and device design |
Table 4: Physical Properties of Covalent Compounds
| Property | Value |
|---|---|
| Melting point | Typically low |
| Boiling point | Typically low |
| Electrical conductivity | Poor |
| Solubility in water | Varies |
Tips and Tricks
- Understand the concept of electronegativity to predict the polarity of covalent bonds.
- Practice drawing Lewis structures to visualize electron-pair sharing.
- Use molecular orbital theory to gain a deeper insight into the nature of covalent bonding.
FAQs
Q1: What is the difference between a covalent bond and an ionic bond?
A: Covalent bonds involve electron sharing, while ionic bonds involve electron transfer, resulting in net charges on the ions.
Q2: Are covalent bonds always nonpolar?
A: No, covalent bonds can be polar if the electronegativity of the bonded atoms is different.
Q3: How does covalent bonding affect the properties of materials?
A: Covalent bonding typically results in materials with low melting and boiling points, poor electrical conductivity, and varying solubility in water.
Q4: What are some examples of covalent compounds used in everyday life?
A: Water, carbon dioxide, and methane are common examples of covalent compounds.
Q5: How can covalent bonding be used in future applications?
A: Covalent bonding can be leveraged in emerging fields such as nanotechnology and bioelectronics through the design of new materials and devices.
Q6: What is a creative new word to describe the process of discovering new applications for covalent bonding?
A: “Covalentiation” conveys the concept of utilizing covalent bonding principles for novel applications.